Optical Tweezers in Microfluidics for Cell Handling: Overview
Can Light Be Used to Manipulate Cells?
The 2018 Nobel Prize winners in Physics proved “YES”. During the past few years it has been shown that both coherent light (laser) and in-coherent light (ambient type light), could be used to manipulate biological cells. The most recent advances in micromanipulation tools and microfluidics have opened up new opportunities in the single cell analysis area. Now, behavior of individual cells can be interrogated and investigated precisely, while in the past they had to be aggregated or averaged. This article summarizes common optical microfluidic manipulation techniques such as optical tweezers, holographic optical tweezers, and optically induced dielectrophoresis.
Introduction to Microfluidic Optical Tweezers
Traditional cell studies are conducted by analyzing statistical responses of large population of cells. This bulk approach fails to study physiological behaviors of those single cells who deviate substantially from the averages[1]. Fortunately several novel Optical Tweezers are under development to get the hold of the single cells. The current optical manipulation techniques can be classified into two major categories. The first category uses direct optical forces generated by optical gradient, or scattering. The second category relates to indirect optical approaches, such as optically induced dieletrophoresis.
The major advantages of optical approaches over other single-cell manipulation tools, such as acoustophoretic or magnetophoretic tweezers, are: a) flexibility, and b) less need to specially designed physical substrates. The flexibilities of the optical approaches allow real-time reconfiguration of the tweezers. For example, the optical patterns for cell manipulation can be dynamically adjusted to switch between various functions, such as isolation or transportation. On a flip side, low throughput is the main drawback of optical approaches for cell handling. Typical optical manipulators have working areas of less than a square millimeter due typical optical microscopy limitations. Although few recent research has been able to conduct highly parallel single-cell manipulation over hundreds of square millimeter, microfluidic chips with massively parallel manipulation capabilities are needed to reduce the operational cost for commercial adoption and success of the technology.
How Direct Optical Forces Are Generated, and Used to Manipulate Cells?
Direct optical force is created when photon energy is directly converted to mechanical force through an optical phenomenon such as refraction, via gradient or scattering of light. The optical tweezers using this strategy are three-dimensional optical traps that generate force on the order of 1-100 pico-Newtons, on 1-10µm particles [2]. Direct optical tweezers may use a single tightly focused laser beam to pass through dielectric particles, in which the refraction index difference between the particle and surrounding medium induces an optical gradient force. When the refraction index of the dielectric particle is larger than the surrounding medium, the dielectric particles move toward the focal point where light intensity is higher.
This figure illustrates how a dielectric particle being trapped by an optical tweezer. 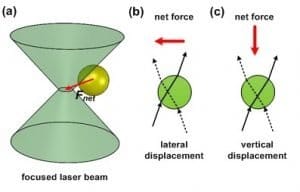 (a) When a laser beam is tightly focused through a high numeric aperture (N.A.) optical focusing system, the dielectric particle could move toward the focal point. (b) If the focal point is positioned on one side of the dielectric particle, the dielectric particle experiences lateral net force. (c) If the focal point is positioned at the bottom of the dielectric particle, the dielectric particle experiences a net force pointing down to the focal point.
(a) When a laser beam is tightly focused through a high numeric aperture (N.A.) optical focusing system, the dielectric particle could move toward the focal point. (b) If the focal point is positioned on one side of the dielectric particle, the dielectric particle experiences lateral net force. (c) If the focal point is positioned at the bottom of the dielectric particle, the dielectric particle experiences a net force pointing down to the focal point.
The difference in refraction index between particle and surrounding medium, along with the optical power of the trap, contribute to how tightly the particle is trapped. Typical light intensity of a single-beam optical tweezer is in the range of 106 Watt/cm2. To achieve these optics, the high numeric aperture (N.A.) objective lenses provide sufficiently high gradient in the light intensity. The high intensity of the light itself, on the other hand, could damage cells [3]. The damage threshold varies for different types of cells. It also relates to the amount of energy absorbed, and to the light wavelength. In practice, the damage threshold is quantified by measuring cell cloning efficiency, where cloning efficiency greater than 75% is considered safe[4].
How to increase throughput?
Throughput of single-beam optical tweezers can be improved by using multiple laser beams to generate several optical traps simultaneously. The multiplication of laser beams can be done by splitting a single beam to multiple locations by using acousto-optical deflectors (AODs)[5]. Diffraction efficiency in this approach is around 80%, which could limit the available power for trapping (Fig. (a)). Another method for increasing throughput is using scanning mirrors. These mirrors scan the working area at frequency of up to several kHz for trapping hundreds of dielectric particles in parallel (Fig. (b)) [6]. another type of laser beam splitting techniques are explored for holographic optical tweezers (HOT) (Fig. (c)). 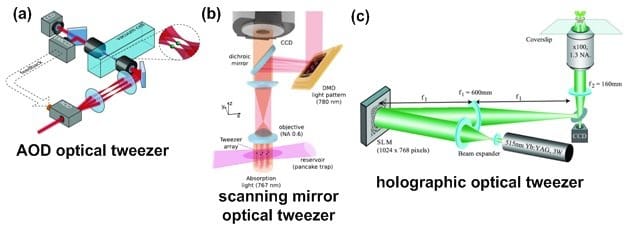 In HOT, a diffraction pattern splits a single laser beam into several different beams for simultaneous and parallel cell manipulation. To control the HOT traps in real-time, spatial light modulators (SLMs) are used to generate holograms created by computer [7]. Between all these methods, HOTs have demonstrated larger working area in the order of few hundred square millimeters. These parallel optical tweezers can be used for the formation of microscale scaffold for microfluidic based tissue engineering or programmed cell cluster formation [8].
In HOT, a diffraction pattern splits a single laser beam into several different beams for simultaneous and parallel cell manipulation. To control the HOT traps in real-time, spatial light modulators (SLMs) are used to generate holograms created by computer [7]. Between all these methods, HOTs have demonstrated larger working area in the order of few hundred square millimeters. These parallel optical tweezers can be used for the formation of microscale scaffold for microfluidic based tissue engineering or programmed cell cluster formation [8].
Optical tweezers can be integrated with imaging and image based cell sorting
The optical nature of these tweezers enable them to be used in conjunction with imaging for applications such as cell sorting. For example, a camera or fluorescence detector can be installed in the optical paths in the microfluidic device. Once the targeted cells are detected by the imaging component of the optical system, the optical tweezers are actuated to deviate the flow direction of those cells to the desired on-chip locations or to desired flow trajectories [9, 10]. Although parallel and dynamic optical tweezers have been used in continuous-flow cell sorting, the throughput of less than 10 cells per second is not impressive enough for adoption of the technology for cell sorting applications.
Optical Scattering Force Based Tweezers
Other than gradient forces, optical scattering force is also used for single-cell manipulation. Optical scattering force works by pushing dielectric particles or cells along the direction of a laser beam. Single-cell trapping was demonstrated by using dual beams from embedded optical fibers facing each other in a microfluidic channel[11]. Single-cell extraction out of microwells for further downstream analysis or disposal was also demonstrated using this approach[12].
How Indirect Optical Forces Are Used for Cell Manipulation?
Another approach for using light for cell manipulation uses indirect optical forces where the driving forces are generated by transduction of light, via mechanisms such as optoelectronic effects.
For example, Optoelectronic Tweezer (OET) is a device that has a multilayer structure. The structure includes: a) A thin layer of transparent electrode made by depositing indium-tin oxide (ITO) on glass. B) A thin layer of photoconductive material deposited on solid or flexible substrate. And C) fluidic layer in between. The fluidic layer allows passage of target cells. An alternating current (AC) signal is applied between the top and bottom layers. When a real-time programmable optical pattern is illuminated on the photoconductive layer, a non-uniform electric field is induced for dielectrophoretic-based cell manipulation[13, 14]. To do so the impedance of the photoconductive layer, made of amorphous silicon (a-Si:H) or a photo-transistor, varies under light illumination. When the light hits the photoconductive layer, the impedance of the illuminated area is lowered significantly, resulting in a significant voltage drop across the liquid layer. This enables optically defined electrodes or “virtual electrodes” in the photoconductive layer.
This figure compares traditional OET (a)[13], with photo-transistor OET (b) [14]. 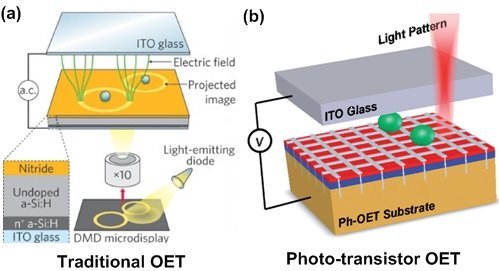 The major difference is that the conductivity of (b) in its illuminated state (on-state) is significantly higher than (a). Thus, (b) is able to operate in regular physiological buffer with liquid conductivity of 1~2 S/m. On the other hand, (a) can only operate in low conductivity buffer with liquid conductivity of 10-6~10-2 S/m. This means that cells have to be re-suspended into such low conductivity isotonic buffer before OET operation risking physiological damage.
The major difference is that the conductivity of (b) in its illuminated state (on-state) is significantly higher than (a). Thus, (b) is able to operate in regular physiological buffer with liquid conductivity of 1~2 S/m. On the other hand, (a) can only operate in low conductivity buffer with liquid conductivity of 10-6~10-2 S/m. This means that cells have to be re-suspended into such low conductivity isotonic buffer before OET operation risking physiological damage.
Because the light patterns shined on the photoconductive layer can be controlled in real-time, the induced virtual electrode pattern can take a desired shape at any given time. This fundamentally eliminates need for challenging micro-electrode fabrication. Furthermore, the light intensity required for OET is only in the range of 10-3 to 1 Watt/cm2. Compared to optical tweezers, this is at least 106 times different, which obviously relaxes the need for high-power laser or focusing optics. Similar to direct optical force methods; OET may allow visualizing the manipulated cells in microfluidic channels. OET is a fast growing topic with many active researches in the areas of cell sorting [16, 17], single-cell manipulation[13, 14], single-cell measurement [18, 19], electrorotation [20], and electroporation[21].
References and Further Reading
- Jammes, F.C. and S.J. Maerkl, How single-cell immunology is benefiting from microfluidic technologies. Microsystems & Nanoengineering, 2020. 6(45).
- Ashkin, A., et al., Observation of a single-beam gradient force optical trap for dielectric particles. Optics Letters, 1986. 11(5): p. 288-290.
- Dholakia, K. and P. Reece, Optical micromanipulation takes hold. nanotoday, 2006. 1(1): p. 18-27.
- Liang, H., et al., Wavelength dependence of cell cloning efficiency after optical trapping. Biophysical Journal, 1996. 70(3): p. 1529–1533.
- Endres, M., et al., Atom-by-atom assembly of defect-free one-dimensional cold atom arrays. Science, 2016. 354(6315): p. 1024-1027.
- Wang, Y., et al., Preparation of hundreds of microscopic atomic ensembles in optical tweezer arrays. npj Quantum Information, 2020. 6(54).
- Leach, J., et al., Manipulation of live mouse embryonic stem cells using holographic optical tweezers. Journal of Modern Optics, 2009. 56(4): p. 448–452.
- Kirkham, G.R., et al., Precision Assembly of Complex Cellular Microenvironments using Holographic Optical Tweezers. Scientific Reports, 2015. 5(8577).
- Jákl, P., et al., Optical sorting of nonspherical and living microobjects in moving interference structures. Optics Express, 2014. 22(24): p. 29746-29760.
- Landenberger, B., et al., Microfluidic sorting of arbitrary cells with dynamic optical tweezers. Lab on a Chip, 2012. 12(17): p. 3177-3183.
- Lincoln, B., et al., Reconfigurable microfluidic integration of a dual-beam laser trap with biomedical applications. Biomedical Microdevices, 2007. 9(5): p. 703-710.
- Kovac, J.R. and J. Voldman, Intuitive, Image-Based Cell Sorting Using Optofluidic Cell Sorting. Analytical Chemistry, 2007. 79(24): p. 9321-9330.
- Chiou, P.Y., A.T. Ohta, and M.C. Wu, Massively parallel manipulation of single cells and microparticles using optical images. Nature, 2005. 436: p. 370–372.
- Hsu, H.-y., et al., Phototransistor-based optoelectronic tweezers for dynamic cell manipulation in cell culture media. Lab on a Chip, 2010. 10(2): p. 165-172.
- Dielectrophoresis (DEP) On a Chip: Overview. 2020; Available from: https://www.ufluidix.com/microfluidics-research-reviews/dep-on-a-chip-review/.
- Huang, S.-B., et al., High-purity and label-free isolation of circulating tumor cells (CTCs) in a microfluidic platform by using optically-induced-dielectrophoretic (ODEP) force. Lab on a Chip, 2013. 13(7): p. 1371-1383.
- Ohta, A.T., et al., Motile and non-motile sperm diagnostic manipulation using optoelectronic tweezers. Lab on a Chip, 2010. 10(23): p. 3213-3217.
- Zhao, Y., et al., Measurement of single leukemia cell’s density and mass using optically induced electric field in a microfluidics chip. Biomicrofluidics, 2015. 9(2): p. 022406.
- Neale, S.L., et al. Optoelectronic tweezers for the measurement of the relative stiffness of erythrocytes. in SPIE. 2012.
- Liang, Y.-L., et al., Cell rotation using optoelectronic tweezers. Biomicrofluidics, 2010. 4(4): p. 043003.
- Chih-Hung Wang, a.Y.-H.L., a Hsin-Tzu Kuo, et al., Dielectrophoretically-assisted electroporation using light-activated virtual microelectrodes for multiple DNA transfection. Lab on a Chip, 2014. 14(3): p. 592-601.
Dielectrophoresis uses electrical field for moving particles in Microfluidics.
What does Electrokinetics mean? How it is used in Microfluidics to manipulate cells?