Liver On A Chip: Recent Microfluidics Advances
What is an Organ on a Chip device? What is Liver on a Chip?
Since its inception as a game-changing advance, organ-on-a-chip and tissue chips have attracted significant interest as ideal Microfluidic environments to study human-level physiology [1], [2]. According to cell biologist and bioengineer Donald Ingber who pioneered the technology [1], an organ-on-a-chip has a slightly more complex functionality compared to tissue chips, since they can model key functions of whole organs to specifically investigate activity of interest at the cellular level [3]. Researchers have thus far mimicked the key functional units of more than 10 human organs, including microfluidic organ-on-a-chip models of human liver tissue [4]. Amongst these, the liver-on-a-chip provides a microfluidics in-vitro platform to compartmentalize this complex organ into smaller and simpler cellular subsystems. Liver on chips help to understand higher-order intercellular communication during drug-induced liver injury (DILI) with unprecedented value in basic and applied research [5].
Amongst these, the liver-on-a-chip provides a microfluidics in-vitro platform to compartmentalize this complex organ into smaller and simpler cellular subsystems. Liver on chips help to understand higher-order intercellular communication during drug-induced liver injury (DILI) with unprecedented value in basic and applied research [5].
Design elements of Liver on chip microfluidics devices
The liver plays a key role during metabolism and can regulate the bioactivity of potential pharmaceutical candidates [6]. It affects the pharmacokinetics and pharmacodynamics of potential drug candidates and is vulnerable to damage by toxins [6]. It is important to develop a biologically relevant in-vitro model to mimic the microenvironment and metabolic activities of the liver for pre-clinical investigations of new drug candidates. Lab-based static systems and animal models are not ideal predictors of liver toxicity and liver disease pathogenesis in humans. It is therefore important to test new drugs in human liver tissue constructs prior to administrating it in people to predict and eliminate liver toxicity, safely and effectively [4].
To maintain liver cell functionalities on chip, researchers must meet a few key requirements:
- Cultivate adequate cell types including, hepatocytes, stellate cells, Kupffer cells and endothelial cells to form a biomimetic microenvironment [7].
- Re-create multicellular three-dimensional (3-D) architecture with a consistent supply of nutrients and oxygen.
- Form tissue-tissue interfaces, enable vascular perfusion and fluid flow using microfluidic channels to gain controlled insight to cellular behavior [4].
Recent Microfluidics Advances in Liver on Chip Research
To this date several Liver on a Chip designs and devices have been investigated by microfluidics researchers [4]. This short review will summarize three recent advances in the state-of-the-art chip-based liver models and their technical design that cleverly combines engineering and biology.
Reproducing human and cross-species drug toxicities on a liver-chip
Published on Science Translational Medicine, three separate species-specific microfluidic chips were engineered and lined with living rat, dog, and human hepatic cells constructed using microfluidic organ-chips [8]. 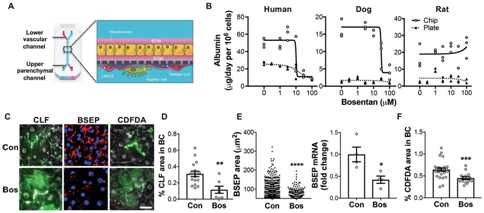 The device contained two parallel microchannels separated by a porous membrane, where the upper parenchymal channel contained primary hepatocytes seeded within an extracellular matrix (ECM) sandwich. On the opposite side of the same membrane in the lower vascular channel, the device contained species-specific liver sinusoidal endothelial cells (LSECs) with or without Kupffer cells and/or stellate cells. The microengineered liver-chips maintained liver-specific physiological functions such as albumin secretion, which when compared with conventional static sandwich culture plates were substantially higher for all three species-specific prototypes.
The device contained two parallel microchannels separated by a porous membrane, where the upper parenchymal channel contained primary hepatocytes seeded within an extracellular matrix (ECM) sandwich. On the opposite side of the same membrane in the lower vascular channel, the device contained species-specific liver sinusoidal endothelial cells (LSECs) with or without Kupffer cells and/or stellate cells. The microengineered liver-chips maintained liver-specific physiological functions such as albumin secretion, which when compared with conventional static sandwich culture plates were substantially higher for all three species-specific prototypes.
The physiological relevance of the dual-cell chips was understood by investigating the drug-metabolizing capacity of the hepatocytes; by measuring the enzyme activities of three key cytochrome P450 (CYP) isoforms (CYP1A, CYP2B, and CYP3A) involved in drug metabolism [9]. The microfluidics experiments were conducted in the presence of a cocktail of probe substrates including phenacetin, bupropion and midazolam . The CYP activities measured in the dual-cell human, rat, and dog chips across 14-days of culture were comparable to, or greater than those exhibited by freshly isolated hepatocytes – an existing gold-standard in pharmaceutical research on liver toxicity [10]. The outcome suggests that the species-specific liver-chip can be used to predict hepatotoxicities for safety and risk assessment. The microfluidic device can aid assess drug-induced liver toxicities and clarify mechanisms of action during drug-induced liver disease in the lab.
A lung/liver-on-a-chip device for acute and chronic toxicity studies
A recent report published on Lab on a Chip describes a method to merge 3-D in vitro models with multi-organ-on-a-chip technology to allow crosstalk between different organs during the in vitro assessment of a chemical compound [11]. A multi-organ-on-a-chip model can combine several systems into one to adequately monitor the metabolic pathway and the potential toxicity, or side-effects of metabolites [12]. The lung/liver-on-a-chip was developed in a single circuit, alongside normal human bronchial epithelial cells cultured at the air-liquid interface with HepaRG™ liver spheroids. The microfluidic device offered a new platform to study the toxicity of inhaled aerosols, and to show the safety and efficacy of new drug candidates. By culturing human primary living cells at the air-liquid interface, a 3-D epithelium could be formed to closely mimic the human lung epithelium [13]. Inhaled aerosols and chemical compounds were absorbed by the lung tissue and partially metabolized by the localized cytochrome P450 enzymes, however, the liver can comparatively express a larger cohort of CYP enzymes highlighting its role during compound metabolism [9].
This prototype microfluidics chip contained four parts including a fluidic plate, pump unit, reservoir plate, and smartphone [11]. 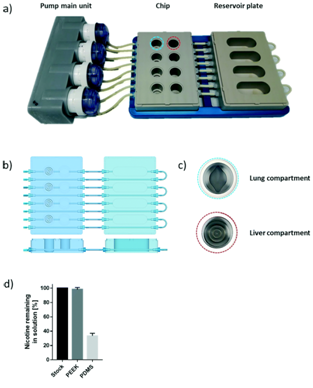 The polyetheretherketone (PEEK) material used for inert medical implants was employed to develop the fluidic and reservoir plate. The fluidic plate had four circuits containing two wells connected by a microfluidic channel with a minimum volume. For long-term culture, the fluidic plate could be connected to a second reservoir plate to increase the total volume of the chip. The pump unit also contained four peristaltic pumps and four Bluetooth controllers located in a sealed waterproof box.
The polyetheretherketone (PEEK) material used for inert medical implants was employed to develop the fluidic and reservoir plate. The fluidic plate had four circuits containing two wells connected by a microfluidic channel with a minimum volume. For long-term culture, the fluidic plate could be connected to a second reservoir plate to increase the total volume of the chip. The pump unit also contained four peristaltic pumps and four Bluetooth controllers located in a sealed waterproof box.
The microfluidic chip can be seamlessly connected to the pump unit to control the flow-rate and flow-direction in each circuit via the Bluetooth connection. The platform showed the effects of metabolites on the lung and the impact of inhaled components on the liver. The microfluidics device is suited to assess the compound toxicity pathway and to test the bioavailability of potential drugs administered through different routes to form more sensitive, safe and efficient drug candidates.
A Scaffold-Free Liver-On-A-Chip
In this design published on Advanced Materials, a physiologically relevant liver model was investigated for predictive drug-induced liver injury (DILI) in vitro [14]. The concept of DILI is difficult to assess when developing drugs, since most in vitro methods cannot recapitulate the complex, multifaceted physiology of the liver long-term. This Liver on a chip microfluidics prototype was developed via biomimetic reconstruction of tissue hierarchy without exogenous scaffolds by introducing primary hepatic stellate cells (HSCs) to secrete a physiologically relevant extracellular matrix (ECM). Using microengineering techniques the assembly of primary liver cells (PLCs) could be controlled into an organotypic hierarchy.
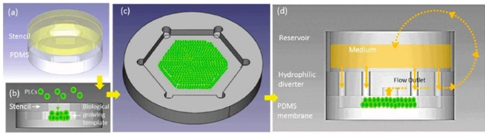
The multi-layered PLCs were deposited on a micro-patterned hydrophobic polydimethylsiloxane (PDMS) membrane through a stencil, to create a biological growth template of hexagonal contour, followed by micro/macro-circulation with a hydrophilic diverter to provide PLCs with vertical cell anchorage. The Liver on Chip device mimicked liver specific functions including the synthesis of albumin and urea, which remained significantly higher than in conventional Petri dish cultures. The setup successfully retained the cytochrome P450 3A4 (CYP 3A4) activity for weeks compared to control cultures [15].
References and Further Reading
- Huh D. et al. Reconstituting Organ-Level Lung Functions on a Chip, Science
- Low L.A. et al. Organs-on-chips: into the next decade, Nature Reviews Drug Discovery
- Bhatia S.N & Ingber D.E. Microfluidic organs-on-chips, Nature Biotechnology
- Singhvi R. et al. Engineering cell shape and function, Science.
- Moradi E. et al. Microfluidic organ-on-a-chip models of human liver tissue, Acta Biomaterialia.
- Trefts E. et al. The liver, Current Biology
- Clark A. M. et al. A liver microphysiological system of tumor cell dormancy and inflammatory responsiveness is affected by scaffold properties, Lab Chip.
- Jang K. J. et al. Reproducing human and cross-species drug toxicities using a Liver-Chip, Science Translational Medicine.
- Zanger U. M. & Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation, Pharmacology & Therapeutics
- LeCluyse E. L. Isolation and culture of primary human hepatocytes, Methods in Molecular Biology.
- Bovard D. et al. A lung/liver-on-a-chip platform for acute and chronic toxicity studies, Lab on a Chip.
- Materne E. M. et al. The multi-organ chip–a microfluidic platform for long-term multi-tissue coculture, Journal of Visual Experiments: JOVE.
- Karp P. H. et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures, Methods in Molecular Biology.
What is Organ on a chip and why it is important.
How Liver on a chip microfluidic devices are used for toxicity screening.