Heart-on-a-Chip: a novel microfluidic approach for cardiac research
There are three sectors at which microfluidics has been revolutionizing; diagnostics, disease modelling, and therapeutic approaches. Upon its advent, microfluidics has influenced and boosted many aspects of biological research domains. Cardiac research has not been an exception in this regard. The cardiac tissue engineering field has been hit by two revolutionizing events; the advent of microfluidic technology and induced pluripotent stem cell technology. Prior experience of researchers in cardiac tissue engineering has been a strong driving force to move the research in this area forward and quickly fabricate biologically relevant cardiac organ-on-a-chips called heart-on-a-chips. The combination of these two has empowered researchers to recreate cardiac tissues from patient-specific models in highly spatiotemporally controlled microfluidics microenvironments for disease modelling and diagnostics as well as therapeutic approaches.
What is a Heart-on-a-Chip?
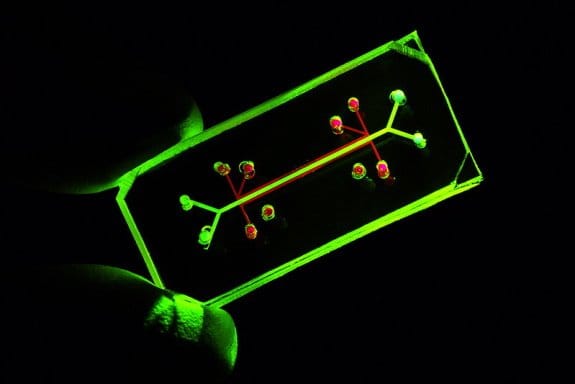
A heart on a chip is a cell culturing microfluidic device to create a highly controlled microfluidics environment to mimic the natural habitat of the heart cells. The heart-on-a-chip devices consist of multiple microchambers and microchannels imprinted on a layer of a polymer that is sealed by bonding to another material, normally a glass slide. Mostly polydimethylsiloxane (PDMS) is used as the polymeric material due to its desirable properties such as transparency and biocompatibility to fabricate these microfluidic devices.
The microchambers and microchannels are used to house the cells and deliver the reagents such as growth factors, cytokines, and nutrients to them. Often the features of a microfluidic chip are coated with biological reagents such as ECM-derived hydrogels to enhance the adhesion of the cells to the surface. Depending on the application, sometimes interdigitated electrodes or a microelectrode array can be printed on the glass surface. The microchambers are lined with patient-specific iPSC derived or primary heart cells and controlled via microchannel, microvalves, and actuators and monitored via biological and digital sensors as well as imaging devices.
The nutrition transport and waste discharge are usually controlled via a continuous or intermittent flow in the main and side microchannels. Pressure and flow sensors are very common in microfluidic devices and can be used for real-time monitoring of the cells. Additionally, the microfluidic channels and microchambers can be coated with ECM-derived hydrogels and enzymes/proteins for enhancing the cell adhesion as well as monitoring the dynamics of changes in the cell culture.
Electrical and mechanical components can be added to the microfluidics heart on a chip to tailor the chip according to the electrophysiology and mechanobiology of the experiment as well as better mimic the in vivo conditions. These electrical and mechanical actuators can serve multiple purposes. For instance, the different electrodes can e placed in the chip both for stimulation of the cells and as a readout system.
Electrical stimulation of cardiomyocytes in Microfluidics heart-on-a-chip platforms
Cardiac muscle cells are known to express strong electrophysiological responses in vivo. Electrical signaling has an important role in tissue development, maintenance, and regeneration. Heart-on-a-chip devices that employ electrodes serve two purposes. They either use the electrodes for stimulating the cells or use them as readout tools for measuring the electrical response of the cells to a particular stimulation.
Electrical stimulation is normally conducted via electrodes that are in contact with the cells. The electrical signal can be applied to the bulk or to the cells at the single-cell resolution. It is common to employ a pair of electrodes and connecting it to the microfluidics device for applying the electrical field to the bulk of the cells. This method is simple lacks high resolution. A microelectrode array is used in cases where cells need to be excited individually. A microelectrode array (MEA) is an array of electrodes containing tens to thousands of microelectrodes. The MEA is manufactured via a lithography process that enables very fine electrodes at the micron-scale size to be printed on the substrates.
What makes a Heart-on-a-Chip important?
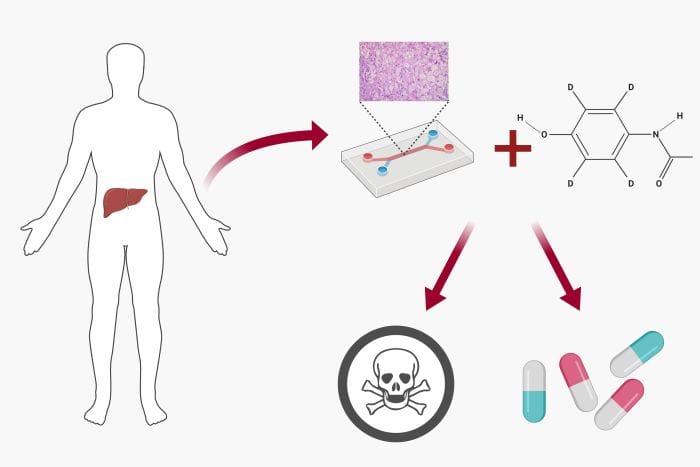
The benefits of using heart-on-a-chips for research and drug development are manifold. Drug-induced cardiotoxicity is a significant issue in drug development pipelines. A considerable amount of drug failures and recalls are due to cardiotoxicity. Effective and accurate in vitro microfluidics models can be an important tool for predicting cardiotoxicity. More advanced models such as heart-on-a-chip devices are able to impose a paradigm shift to the current trend. Conventional 2D models lack in modelling cell-cell and cell-ECM communication properly while the response of animal models’ organs are not precisely projectable to the human counterparts. An original uFluidix article.
Read more on how microfluidics organ on a chips are used to reduce the drug development failure rate and for personalized medicine.
Explore the different aspects of designing a microfluidics organ on a chip. Learn about the cell source, flow dynamics, and the structure of the organ on a chips.
There are questions that should be addressed before moving the organ on a chips to the mainstream applications. Learn more about these aspects of microfluidics organ on a chips.
Discover the advancements of the microfluidics approaches for recapitulating the brain function on brain-on-chip devices.
In a liver-on-a-chip, a highly controlled microfluidics cell culture environment is employed for drug toxicity screening and metabolism analysis.