Microfluidic approaches for immobilizing and positioning C. elegans
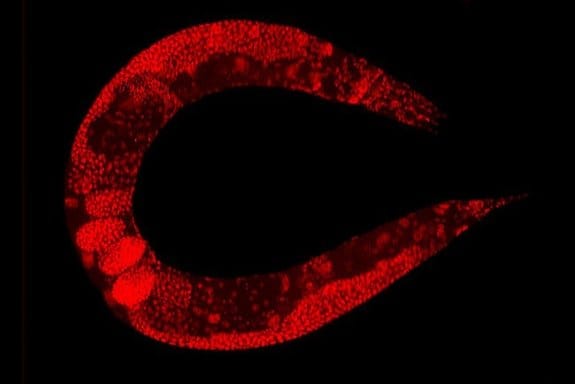
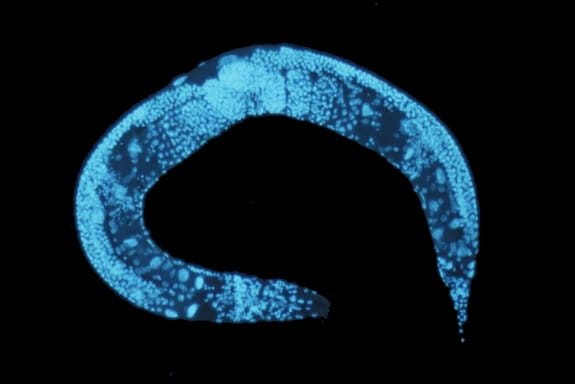
C. elegans is a transparent soil-dwelling nematode. The transparent body of the worm allows real-time imaging and analysis of molecular and cellular events in the body. A protein of interest can be fluorescently tagged in vivo and observed. However, screening the dynamic processes in C. elegans requires precise positioning or immobilizing of the worm. Conventionally, the worms were glued or anesthetized that can disrupt the normal functionality of the worm and lead to false results. Moreover, in many cases, it would be very difficult to recover the worm after it has been glued or anesthetized which impedes further screening. Microfluidics technology offers anesthetic-free and glue-free control of the worm inside the chips which allows screening the dynamics and imaging of the worm. Depending on the specifications of the experiment, any of the following approaches can be employed. These designs of microfluidic chips, which often use PDMS, can be combined with side microchannels to stimulate the worms electrically, mechanically, optically, or chemically while being immobilized. For certain applications, it is required to move the worm to a specific place in the device. The microfluidics based positioning techniques mentioned in the next part can be helpful when designing a positioning tool for a C. elegans study.
Immobilization Methods using Microfluidics
The optical transparency of the microfluidic devices along with the high gas permeability and biocompatibility makes them an ideal choice for C. elegans manipulation and immobilization. The worms can simply be released from the chips for further analysis.
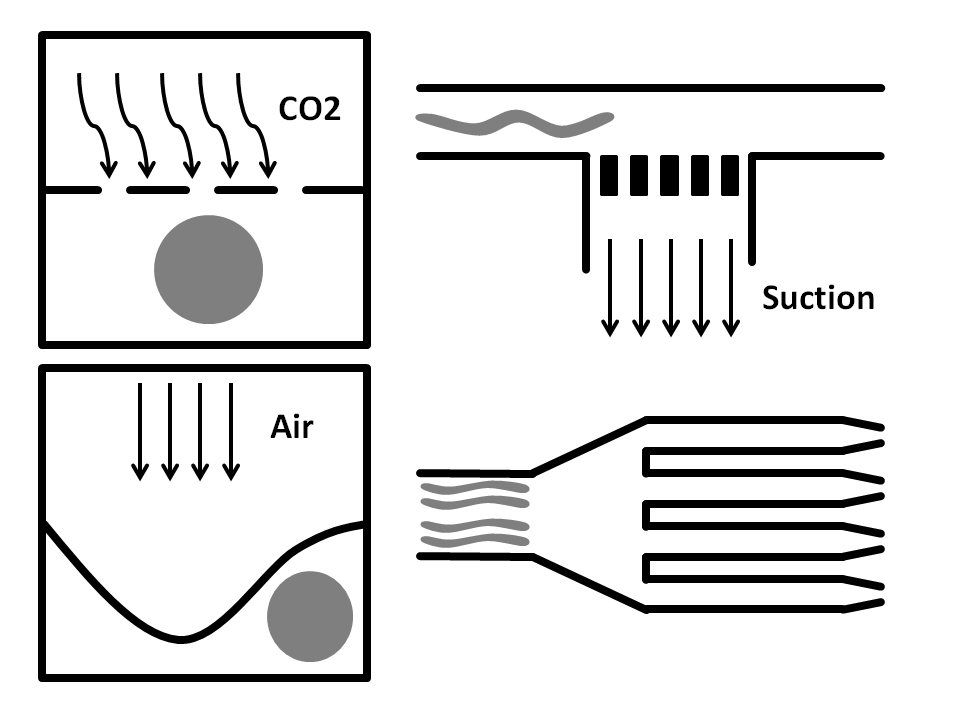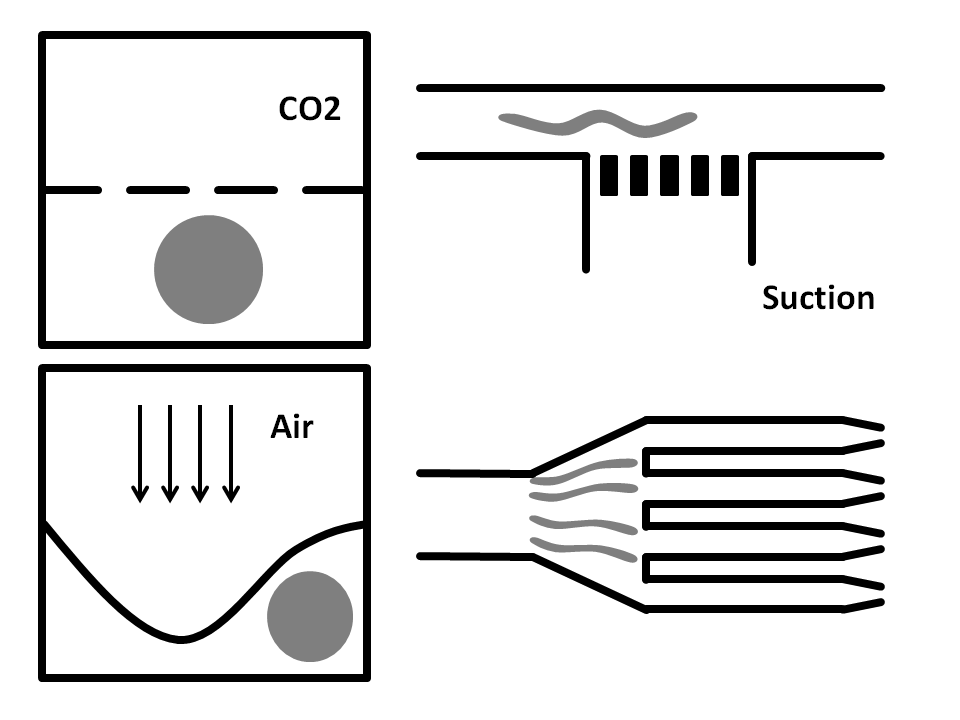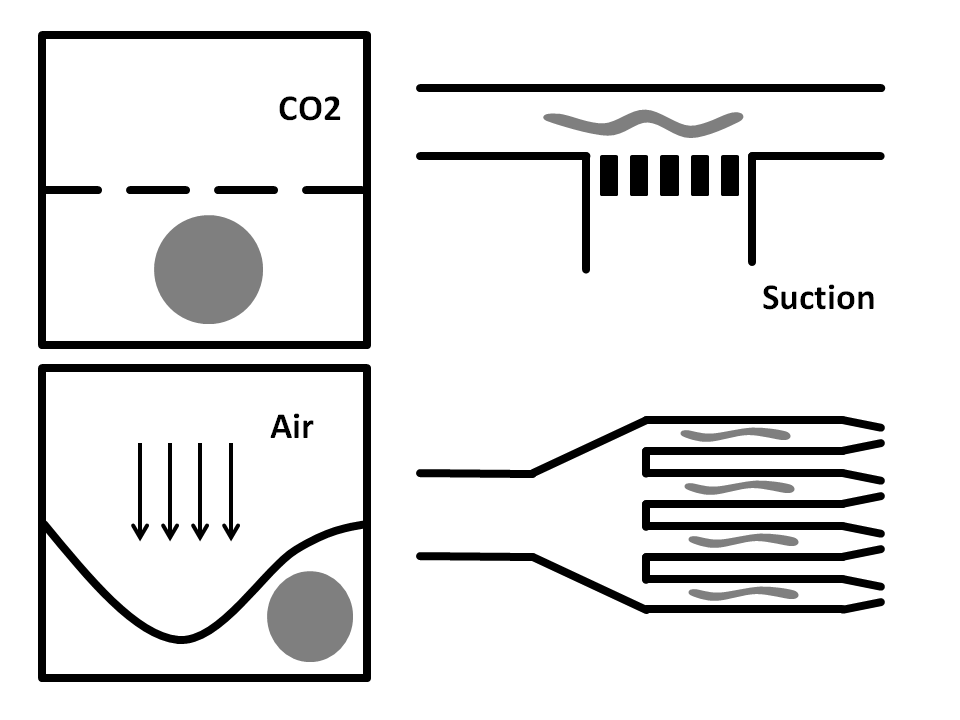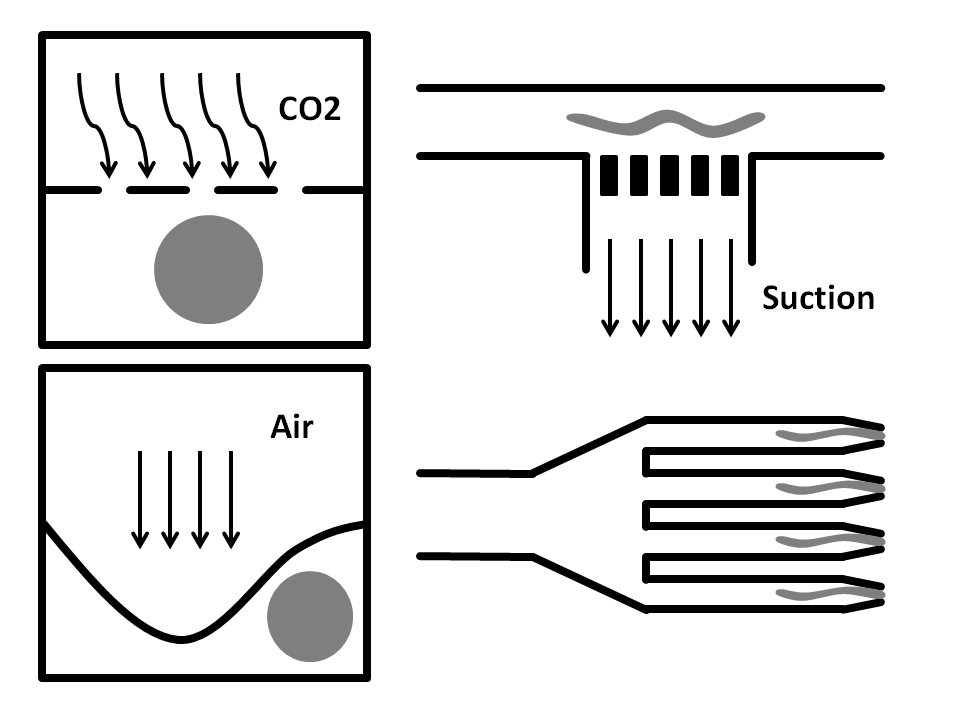
Side-Suction: The worms are flown through a straight microchannel where they are sucked towards the inner wall caused by the side-suction. This microfluidics based method allows for single worm capturing and analysis.
Tapering: This method is suitable for high-throughput screening. Here, worms go through tapered microchannels were they get stuck in the channel due to back pressure caused by the flow.
Air pressure: Here, the microfluidic chip is fabricated from three layers. The bottom layer is where the worm solution flows through. The top layer is full of air and is used to compress the thin middle layer to immobilize the worm. It has been shown that this method does damage the worms.
CO2 Immobilization: In here, the microfluidic chip has three layers. The worms run through the bottom layer. CO2 is run through the top layer and protrudes through the thin porous middle layer. Exposure to CO2 immobilizes the worm for 2-3 hours.
| Side suction | Tapering | CO2 exposure | Air pressure | |
| Chip layers | Single-layer | Single-layer | Two layers | Two layers |
| Throughput | Low | High | Medium | Medium |
| Ease of use | Medium | Easy | Medium | Medium |
| Precision | High | Medium | Medium | Medium |
| Rotation | Yes | No | No | No |
| Damages to the worm | Low | Low | Yes in the long run | Yes in the long run |
Microfluidics Positioning Techniques
Electrotaxis: C. elegans is responsive to the electric field. It responds to both the direction and the strength of the electric field. This behaviour is called electrotaxis and can be used to move and position the worms inside the channel. Placing the electrodes in the desired place in the mcicrofluidic device and applying the voltage can help in the voluntarily positioning of the worms.
Chemotaxis: C. elegans is capable of identifying chemical cues via two types of organs located in the head area and the posterior, namely amphids and phasmids. These sensory systems help the nematode to orient itself in the environment. The worm’s response to chemical gradients is helpful for positioning the worms inside microfluidic chips. C. elegans has response mechanisms to various chemicals, odours, and gases that can help the worm identify the food sources. The worm can swim toward or away from a chemical gradient and therefore hold a specific position in the microfluidics chip.
Phototaxis: C. elegans can respond to light in a process called phototaxis. Although C. elegans does not carry photosensing neurons, it can respond to optical cues via different mechanisms. For example, the blue light can induce acetylcholine in the worm which is a neurotransmitter that causes muscle contraction. – A uFluidix original Article.
Further Reading
- A microfabricated array of clamps for immobilizing and imaging C. elegans
- A hybrid microfluidic device for on-demand orientation and multidirectional imaging of C. elegans organs and neurons
- Microfluidics immobilization of physiologically active Caenorhabditis elegans
- CO2 and compressive immobilization of C. elegans on-chip
Read more on how microfluidics technology is employed for sorting and synchronizing C. elegans
Learn more on behavioural and ageing assays performed using C. elegans in microfluidic chips