In 2007, Doug Oliver nearly hit two pedestrians while driving his car, and then turned a corner and almost hit a third. He had not seen the pedestrians at all. A police officer gave him two choices: hand over your driver’s license or see an eye doctor. The doctor gave a chilling diagnosis: “At 45, I was legally blind. I went into shock,” Oliver said.
Oliver was born with good eyesight, but due to a hereditary condition, over a decade he had gradually lost much of his vision. For years his sight had been worsening until he underwent experimental stem cell surgery in a Florida-based treatment study. His vision loss was reversed by that surgery in 2015. “I went from legally blind to legal-to-drive in eight weeks,” said the Nashville, Tenn., man.
Oliver’s own retinal specialist had described his condition as “incurable” in no uncertain words. Yet days after surgery, “I came out of the surgeon’s office and saw the crisp sparkle of cars in the sun parked in the parking lot. And the leaves on the trees.” And a few months later, he saw his grandchildren’s’ faces for the first time.
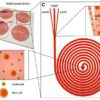
According to the World Health Organization, about 36 million people worldwide are blind and 217 million have vision impairment. Fortunately for them, the list of innovative ways microfluidics technology promises to buttress stem cell treatment for blindness is impressive. Microfluidics engineering has the potential to advance the design of tiny systems and procedures to make stem-cell-generated organoids and tissues-on-chips more functional — more like a real human organ, thus advancing understanding of stem cell effectiveness.
Living organs comprise branched vascular networks, and almost all cells are close to a capillary, for sufficiency. Vascularization that delivers oxygen and nutrients to organoids is necessary; during organoids’ early development they were isolated in lab dishes and were therefore by definition diffusion-limited. Control of microfluids within tissue-chips could help to mimic natural human physiology. This is one way that microfluidic engineering might move stem cells’ capabilities forward.
Microchannels can permit fluid flow at real-life rates. Microfluidic devices in tissues-on-chips can innovatively duplicate essential functions of blood vessels for oxygen flow, while also removing waste.
Although much has been demystified by science in recent decades, much is still unknown about how stem cells work. Microfluidic devices may offer a way to study the decision-making process of stem cells. They can imitate the structural physiology of organs and provide a platform for further study. “The Organoid Revolution” seminars at the World Stem Cell Summit in Miami in January 2018 highlighted new developments.
Stem cells have been successfully used in a number of patient-consented, experimental surgeries, internationally as well as in several U.S. National Institutes of Health-sponsored clinical trials. The need for thorough, prudent painstaking research of the highest quality and maintained at rigorous academic standards — a long, slow process — can be at odds with vulnerable people seeking timely access to treatments for any number of serious conditions — especially those who see that stem cell treatment works for others. Microfluidics engineering has the potential to speed understanding of stem cells’ effectiveness and could potentially shorten approval processes, while simultaneously thwarting unethical use.

Doug Oliver, left, President of the Regenerative Outcomes Foundation, recently interviewed Dr. Francis Collins, Director of the U.S. National Institutes of Health, in connection with the World Stem Cell Summit. The interview is one of Oliver’s “Pioneers of Hope” series.
Credit: Regenerative Outcomes Foundation 2017
The vision was restored for Oliver after he was nearly blind for 11 years. His treatment could have been classified as “unproven” by the FDA, although his outcome has been clinically verified, and he is currently undergoing additional testing at the U.S. National Eye Institute. He considers himself living proof of the game-changing benefits of cell therapy. Organoid systems offer one of the most promising platforms for harnessing stem cells’ power.
Doug Oliver now spends much of his time advocating for patients seeking treatment for vision loss and other serious conditions to access clinical trials that might restore their health. In 2016, he founded The Regenerative Outcomes Foundation, to support outcomes research, inspire hope and raise awareness, and provide resources to help smooth the way for patients navigating the bureaucracy of clinical trials. “I want these treatment trials to be available sooner and to more people who have no hope of treatment in the near future,” he says.
FDA approval of drugs requires evidence they are safe and effective, something that historically has cost drug companies millions of dollars and many years of clinical trials to achieve. Some have proposed even longer approval processes to deter unsafe stem cell practitioners offering “snake oil” treatments.
Oliver believed a process could be designed that struck a balance between access for patients and the need for safe and effective treatments for serious conditions, including vision loss. In 2016, the U.S. Congress agreed and enlisted Oliver to help craft the 21st Century Cures Act. “I wanted these treatment trials to be available sooner and to more people who have no hope of treatment in the near future for blindness,” Oliver says. He guided the Senate HELP Committee in developing FDA procedures that enforce safety while not “suffocating ingenuity the nation needs.”
Ingenuity is exactly where microfluidics research comes in.
Enjoyed this article? Don’t forget to share.

Kathy Jean Schultz
Kathy Jean Schultz is a freelance medical science writer who focuses on medical innovations. She earned a Master’s Degree in Research Methodology from Hofstra University, and a Master’s Degree in Psychology from Long Island University. She is a member of the National Association of Science Writers, and the Association of Health Care Journalists. Her articles about organoids include "Would you trust a 3-D printed mini organ to test your drugs?" and "Stem cells not only slow disease, they come with their own safety test".