08 May New Approaches to Vascularization in Organoid Cultures Using Microfluidic Devices
In tissue engineering, the creation of a vascular network within organoids that can efficiently supply nutrients and oxygen while removing waste is a significant challenge. Most tissues thicker than 400 µm require such a network to prevent necrosis. Traditionally, organoids are often transplanted into host animals to establish a functional blood supply, which is costly and lacks scalability for high-throughput applications like drug screening. Moreover, existing microfluidic chips often fall short of adequately mimicking the complexity of in vivo blood flow, posing a substantial barrier to the study of vascular integration in a controlled environment. Researchers have now developed a microfluidic platform that can culture and vascularize organoids on a chip, effectively bypassing the need for animal hosts.
“Despite rapid advancement in microvascular network systems and organoid technologies, vascularizing organoids-on-chips remains a challenge in tissue engineering. Most existing microfluidic devices poorly reflect the complexity of in vivo flows and require complex technical set-ups. Considering these constraints, we develop a platform to establish and monitor the formation of endothelial networks around mesenchymal and pancreatic islet spheroids, as well as blood vessel organoids generated from pluripotent stem cells, cultured for up to 30 days on-chip.“, the authors explained.
Organoids are three-dimensional cellular constructs that mimic the structure and function of real organs. These tiny, yet complex structures offer incredible insights into organ development, disease progression, and the specific impacts of pharmaceuticals. This new microfluidic device uses a novel design microfabricated from cyclic olefin copolymer—a material chosen for its clarity, chemical resistance, and compatibility with imaging techniques. The proposed microfluidic platform features a series of microchannels that hold organoids securely in place, allowing for the precise delivery of nutrients and the formation of vascular networks around each organoid.
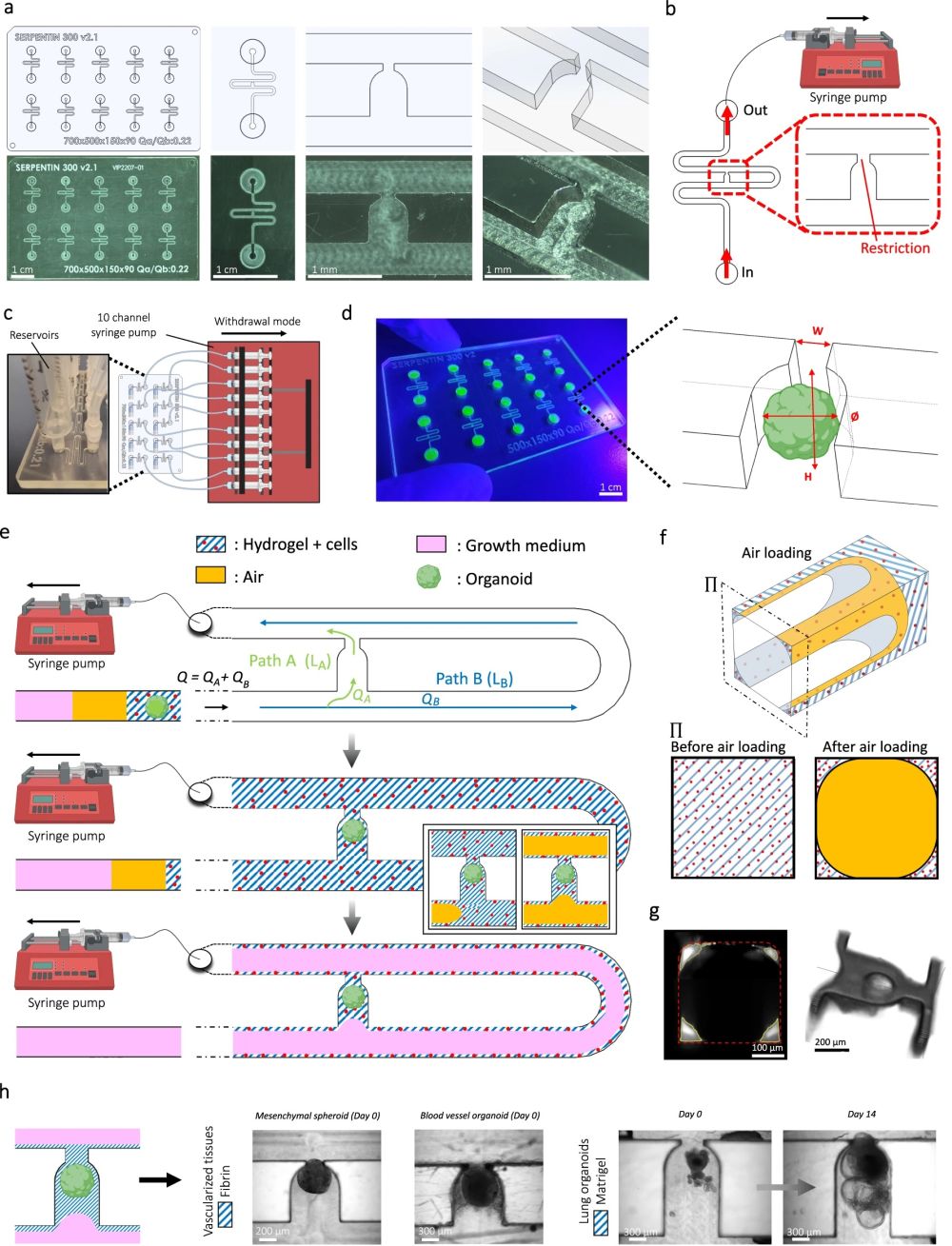
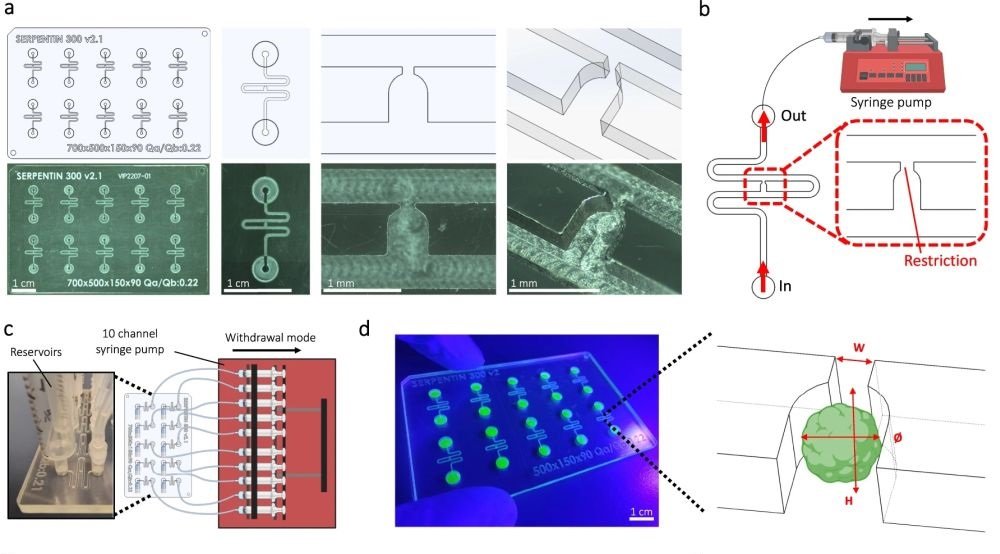
“a Computer-aided design (top) and photographs (bottom) of the microfluidic chip displaying 10 microchannels, with each microchannel featuring a trapping site. b Top view of the microfluidic device. A syringe pump was connected to the outlet of the channel to introduce fluid perfusion. c Schematic diagram and photograph of the parallelization feature of our setup, showcasing 10 microchannels controlled simultaneously. d Photograph of the microfluidic chip and schematic three-dimensional view of the U-cup shaped area functioning as a trap. Here, the trap site is exemplarily occupied by a cell aggregate. e Schematic diagram showing an overview of the loading process. Initially, the hydrogel containing an organoid and HUVEC cells was introduced. Before polymerization of the hydrogel, air was introduced to position the hydrogel and the HUVEC cells. Finally, growth medium was introduced for continuous perfusion of the microfluidic chamber and the trapped organoid. f Schematic 3D and cross-sectional views of the microchannel showing the air loading process and associated hydrogel deposition. g Experimental cross-sectional view (left) of the microfluidic channel showing the hydrogel deposition in the trap and in the channel’s corners and 3D rendering (right), taken with an in-house light sheet fluorescence microscopy set-up. h Representative images of vascular spheroid/organoid cultured in fibrin (left), and hiPSC-derived lung organoids cultured in Matrigel, showing efficient trapping and robust growth over 2 weeks on-chip (right).” Reproduced from Quintard, C., Tubbs, E., Jonsson, G. et al. A microfluidic platform integrating functional vascularized organoids-on-chip. Nat Commun 15, 1452 (2024). under a CC BY 4.0 Attribution 4.0 International license
Microfluidic Innovations
Microfluidic Chip Design: They designed a chip using cyclic olefin copolymer, which has excellent optical properties for microscopy, chemical resistance, and is amenable to mass production. The microfluidic chip includes microchannels that contain specific trapping sites for organoids, allowing precise placement and stable encapsulation of organoids within a hydrogel.
Vascularization Technique: The chip supports the growth of endothelial cells around the organoids, forming functional vascular networks. This is achieved through the strategic design of the microfluidic channels and the hydrodynamic trapping mechanism that positions the organoids in optimal locations for vascularization.
Perfusion System: A continuous flow of medium through the microchannels ensures the delivery of nutrients and oxygen to the organoids and the endothelial networks, mimicking the blood flow found in living organisms.
The implementation of this microfluidic device has led to several impressive outcomes. The vascularized organoids demonstrated improved growth, maturation, and functionality. For example, pancreatic islet spheroids showed enhanced glucose responsiveness—a crucial indicator of their effectiveness for diabetes research. Additionally, the design allows multiple organoids to be cultured and analyzed simultaneously, offering a scalable solution ideal for extensive pharmaceutical testing and research. By providing an alternative method for organoid vascularization, this technology reduces the reliance on animal models, aligning with the increasing demand for ethical scientific practices.
The study demonstrated functional anastomosis between human umbilical vein endothelial cells (HUVECs) and blood vessel organoids (BVOs) on the chip. The integration of these networks was confirmed by CD31 staining, indicating successful vascularization within the microfluidic environment. The average diameter of the HUVEC vessels was measured at about 37 µm in areas upstream and downstream of the BVOs, resembling arteriole-venule sizes in vivo, which suggests physiologically relevant vessel formation.
The vascularized pancreatic islet spheroids on the chip demonstrated improved functionality. Specifically, glucose-stimulated insulin secretion (GSIS) assays showed significant increases in insulin secretion in response to high glucose levels. This result was particularly notable in islets cultured under flow conditions with vascularization, suggesting enhanced beta-cell function due to better mimicry of physiological conditions.
These results collectively underscore the efficacy of the microfluidic platform in creating a physiologically relevant, controlled environment that supports the growth, maturation, and functional enhancement of vascularized organoids. This platform offers significant potential for applications in disease modeling, drug testing, and potentially in regenerative medicine by enabling detailed studies of human tissues in vitro without the need for animal models.
“Our platform now allows us to explore diverse topics, such as organoid lifespan enhancement through vascularization, exposure to drugs, nucleic acids or metabolic stress. The device we have developed also offers the flexibility to vascularize other types of organoids, spheroids, tumoroids, or human tissue explants, as exclaimed in our study by improved glucose responsiveness of islet spheroids.“, the authors concluded.
Figures are reproduced from Quintard, C., Tubbs, E., Jonsson, G. et al. A microfluidic platform integrating functional vascularized organoids-on-chip. Nat Commun 15, 1452 (2024). https://doi.org/10.1038/s41467-024-45710-4 under a CC BY 4.0 Attribution 4.0 International license.
Read the original article: A microfluidic platform integrating functional vascularized organoids-on-chip
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


