28 May Monolayer assembly of barcoded microparticles for multiplexed immunoassays
One of the reasons that some of the microfluidic products that show promise in research settings never find their way to the market lies in the complicated operation procedure. The simpler the microfluidic device the higher the chance of that product being well-received by the market. For this week’s research highlight, we have selected one such advancement. In this communication article published in the Lab On a Chip journal, a research team reported a microfluidic chip that simplifies the assembly of barcoded planar microparticles for multiplexed immunoassay. In the proposed method, the team showed that by using only a pipette, they could assemble a monolayer of fluorescent barcoded particles in a microfluidic chip.

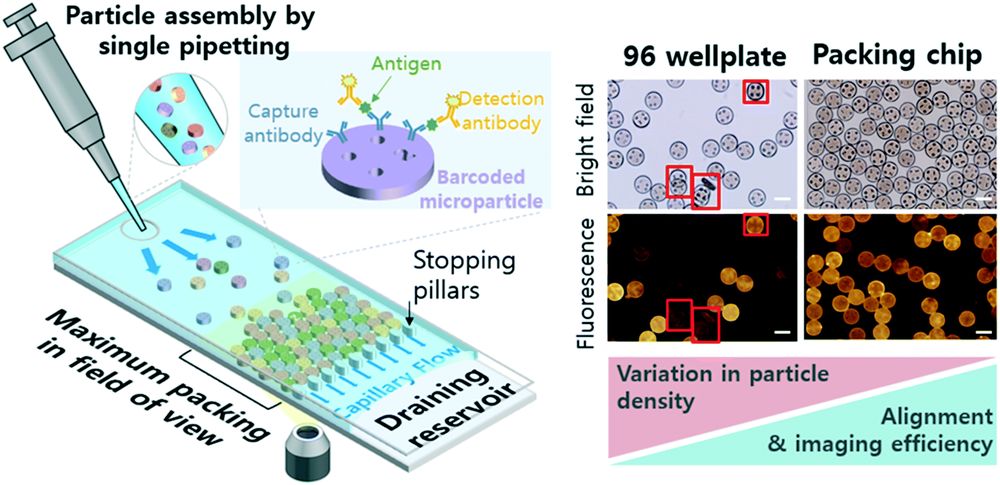
Schematic of a microfluidic, pipetting-based packing of encoded microparticles. Efficient readout of encoded microparticle-based multiplex immunoassay can be done by a pipetting-induced capillary flow-based monolayer assembly of microparticles into a microfluidic chip. The assay readout becomes efficient compared to simple microparticle dispensing on a flat surface (i.e. 96 well plate) because of simultaneous increase of microparticle assembly density and alignment quality. Scale bars are 100 μm. Reproduced from S. Bae, D. Lee, H. Na, J. Jang and S. Kwon, Lab Chip, 2022, Advance Article , DOI: 10.1039/D2LC00174H with permission from Royal Society of Chemistry.
“Barcoded planar microparticles are suitable for developing cost-efficient multiplexed assays, but the robustness and efficiency of the readout process still needs improvement. Here, we designed a one-step microparticle assembling chip that produces efficient and accurate multiplex immunoassay readout results. Our design was also compatible with injection molding for mass production.“, the authors explained.
The proposed microfluidic chip is made with PDMS using conventional microfluidic microfabrication techniques. The microfluidic chip was designed such that it could be operated without the need for pumps and valves. The barcoded microparticles could be guided through the device by a pipetting-induced capillary flow. The stopping pillar downstream of the microfluidic chip ensured that the microparticles did not escape the chip. The microfluidic chip was first optimized to find the channel height that results in a monolayer assembly. Upon optimization, 8 parallel microfluidic channels were placed alongside each other to increase the readout throughput. Fluorescent imaging of the microfluidic chip indicated the successful formation of a monolayer of fluorescent microparticles.
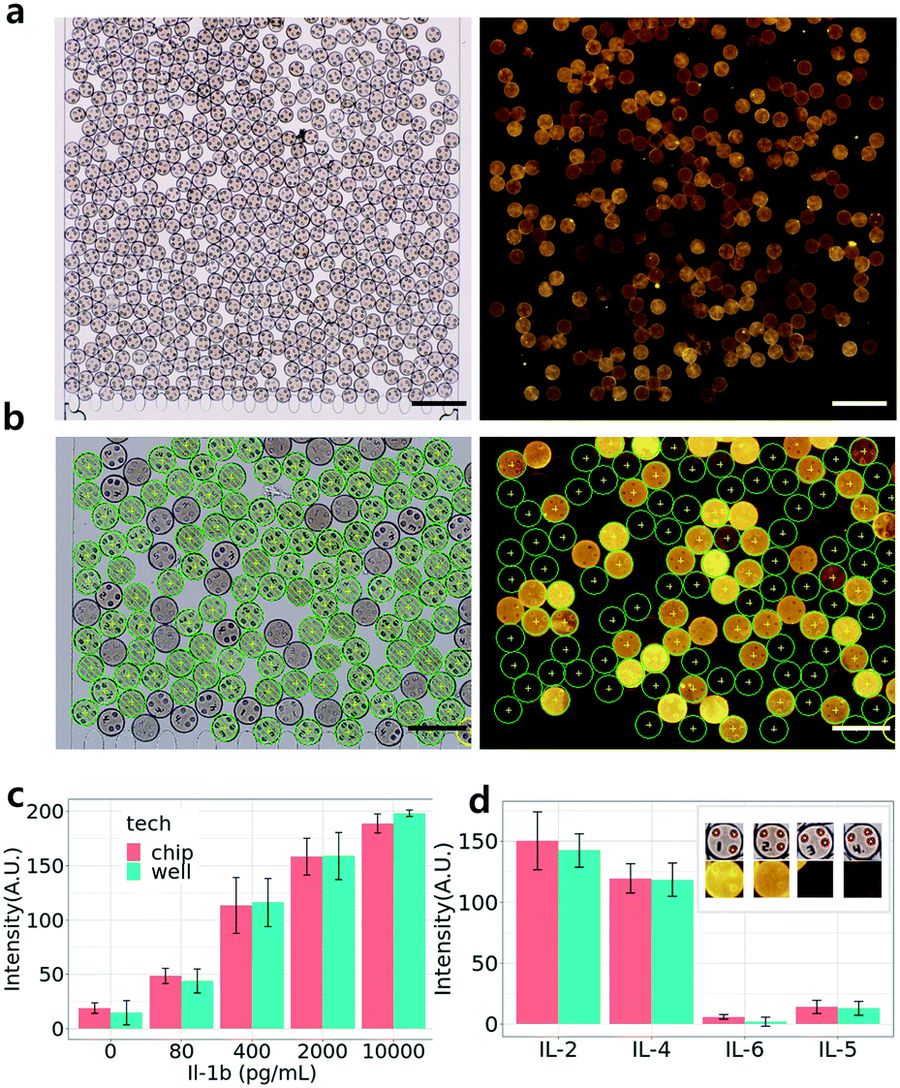
High throughput multiplexed immunoassay using a packing chip. (a and b) Bright field (left panel) and fluorescence (right panel) images of a 4-plex cytokine immunoassay using the microparticle packing chips. As indicated in (b), each microparticle was correctly captured by our image processing algorithm. Scale bars are 400 μm (a) or 200 μm (b). (c and d) Comparison of 5-plex one-pot generated standard curves of IL-1β (c) and 4-plex cytokine detection profiles (d) obtained by either the 96 well plate experiment (blue) or the microparticle packing chip (red). Input antigen concentrations were 10 000 pg mL−1 for IL-2 (code 1), 2000 pg mL−1 for IL-4 (code 2), 400 pg mL−1 for IL-5 (code 4) and 0 pg mL−1 for IL-6 (code 3). Error bars are standard deviations. The number of measurements (N) for each error bar was 27–45 for 4(c) well experiments, 38–135 for 4(c) chip experiments, 130–162 for 4(d) well experiments, and 13–131 for 4(d) chip experiments.. Reproduced from S. Bae, D. Lee, H. Na, J. Jang and S. Kwon, Lab Chip, 2022, Advance Article , DOI: 10.1039/D2LC00174H with permission from Royal Society of Chemistry.
“Other than the chip and a pipette, our strategy doesn’t require cumbersome syringe pumps or other fluidic controlling modules. We expect that this chip design will find utility in point-of-care applications where tools are encouraged to be simple yet have high throughput, and multiplexing capacity is required.“, the authors concluded.
Figures are reproduced were reproduced from S. Bae, D. Lee, H. Na, J. Jang and S. Kwon, Lab Chip, 2022, Advance Article , DOI: 10.1039/D2LC00174H with permission from the Royal Society of Chemistry.
Read the original article: One-step assembly of barcoded planar microparticles for efficient readout of multiplexed immunoassay


