12 Mar Microfluidics-enabled molecular interrogation of mosquito bites
“Mosquito bites transmit a number of pathogens via salivary droplets deposited during blood-feeding, resulting in potentially fatal diseases. Little is known about the genomic content of these nanodroplets, including the transmission dynamics of live pathogens. Here we introduce Vectorchip, a low-cost, scalable microfluidic platform enabling high-throughput molecular interrogation of individual mosquito bites. We introduce an ultra-thin PDMS membrane which acts as a biting interface to arrays of micro-wells. Freely-behaving mosquitoes deposit saliva droplets by biting into these micro-wells. By modulating membrane thickness, we observe species-dependent differences in mosquito biting capacity, utilizable for selective sample collection. We demonstrate RT-PCR and focus-forming assays on-chip to detect mosquito DNA, Zika virus RNA, as well as quantify infectious Mayaro virus particles transmitted from single mosquito bites. The Vectorchip presents a promising approach for single-bite-resolution laboratory and field characterization of vector-pathogen communities, and could serve as a powerful early warning sentinel for mosquito-borne diseases.”
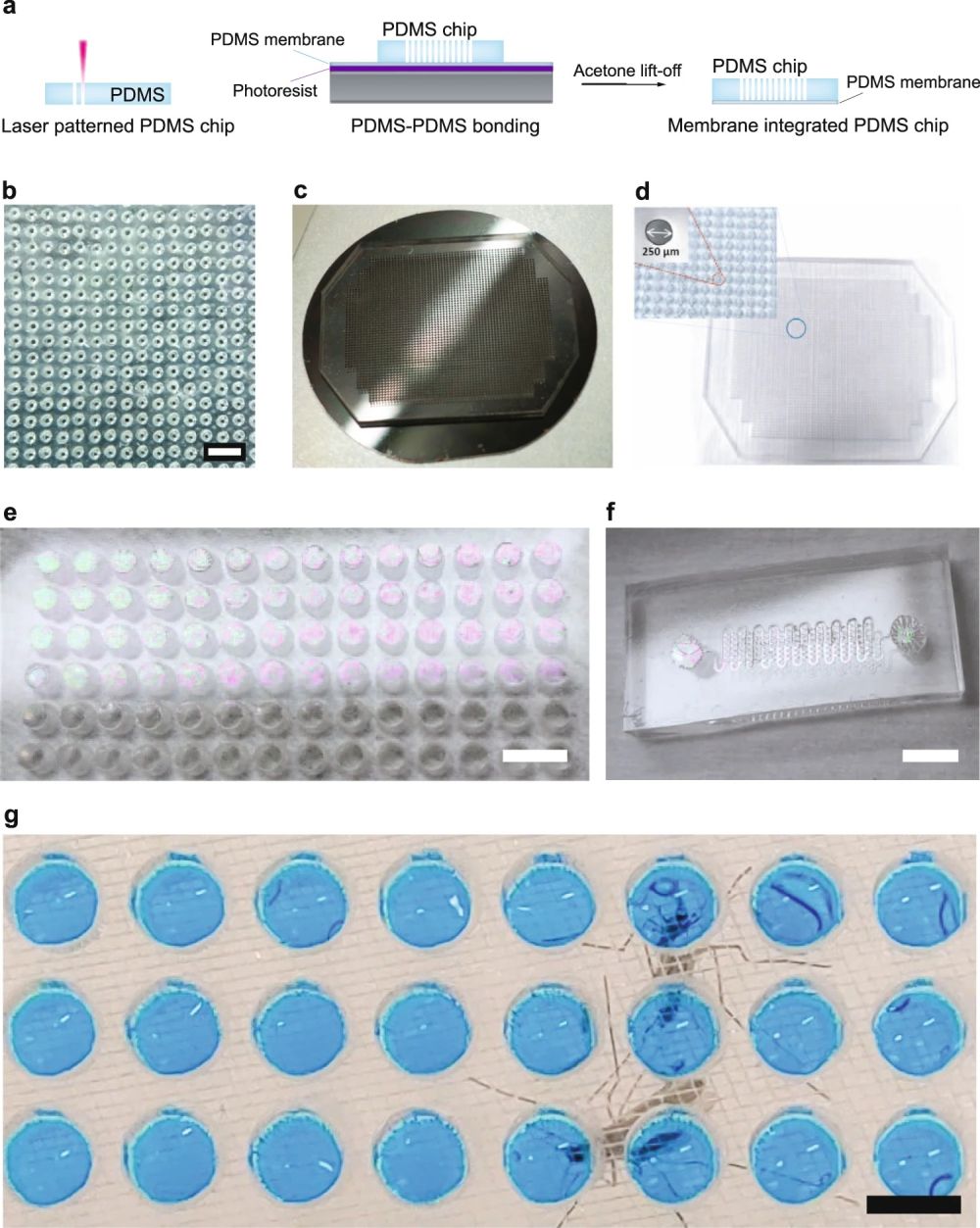
“a Schematic showing the fabrication process of Vectorchip. Laser patterning was used to obtain through-holes in blocks of PDMS. A thin sacrificial layer of photoresist (Microposit™ S1813 G2, DOW Inc., USA) was spread onto a silicon wafer followed by spin-coating a thin film of PDMS on the wafer surface. The laser-patterned body of the chip was then bonded to the thin film of PDMS using plasma-activated PDMS-PDMS bonding. Membrane-bonded chips were obtained by placing the wafer in an acetone bath, where the sacrificial layer of resist was dissolved. b A PDMS block with laser-generated through-holes (diameter 250 μm). c A PDMS chip bonded to a 1.6 μm thin PDMS membrane on a 4-inch silcon wafer. d A Vectorchip with around 3000 wells (dia: 250 μm) is shown. e Chip with well diameter 1.75 mm and a visible PDMS membrane (thickness 1.6 μm). f Microfluidic channel-integrated chips with a PDMS membrane on top. g These wells can be loaded with feeding solution, reaction reagents, and can store mosquito saliva after biting assays. Wells loaded with feeding media (10% sucrose laced with blue food color) shown in the image. Scale bars represent 1 mm in (b), 5 mm in (e), and 2 mm in (f, g).” Reproduced under Creative Commons Attribution 4.0 International License from Kumar, S., Hol, F.J.H., Pujhari, S. et al. A microfluidic platform for highly parallel bite by bite profiling of mosquito-borne pathogen transmission. Nat Commun 12, 6018 (2021).
Figures and the abstract are reproduced from Kumar, S., Hol, F.J.H., Pujhari, S. et al. A microfluidic platform for highly parallel bite by bite profiling of mosquito-borne pathogen transmission. Nat Commun 12, 6018 (2021). https://doi.org/10.1038/s41467-021-26300-0 under Creative Commons Attribution 4.0 International License
Read the original article: A microfluidic platform for highly parallel bite by bite profiling of mosquito-borne pathogen transmission


