28 Jul Microfluidics-enabled analysis of signaling dynamics in single-cells
Abstract
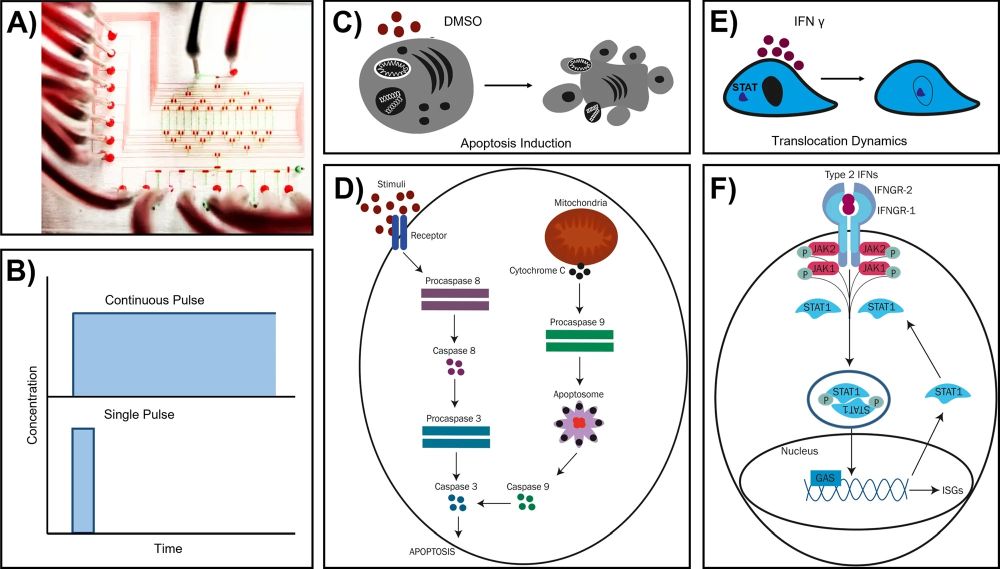
“Microfluidic designs are versatile examples of technology miniaturisation that find their applications in various cell biology research, especially to investigate the influence of environmental signals on cellular response dynamics. Multicellular systems operate in intricate cellular microenvironments where environmental signals govern well-orchestrated and robust responses, the understanding of which can be realized with integrated microfluidic systems. In this study, we present a fully automated and integrated microfluidic chip that can deliver input signals to single and isolated suspension or adherent cells in a precisely controlled manner. In respective analyses of different single cell types, we observe, in real-time, the temporal dynamics of caspase 3 activation during DMSO-induced apoptosis in single cancer cells (K562) and the translocation of STAT-1 triggered by interferon γ (IFNγ) in single fibroblasts (NIH3T3). Our investigations establish the employment of our versatile microfluidic system in probing temporal single cell signaling networks where alternations in outputs uncover signal processing mechanisms.”
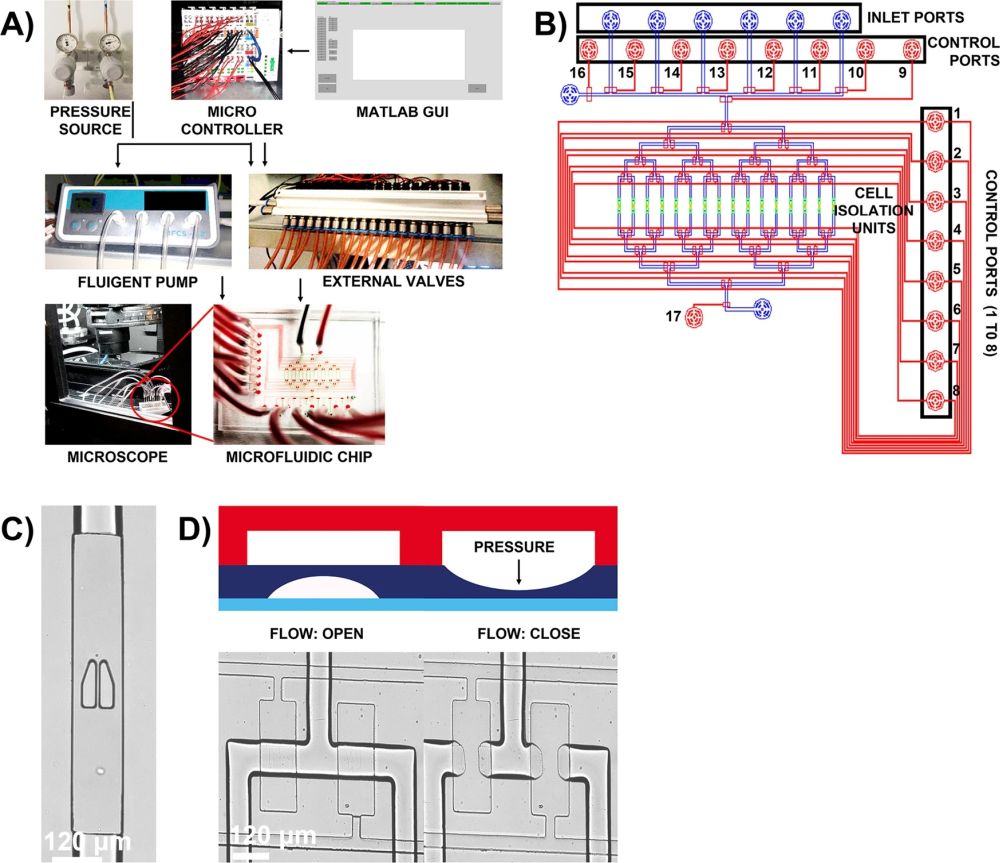
A The complete setup of two-layer microfluidic chip comprises of external pneumatic membrane valves and pressure source, to actuate on-chip pneumatic membrane valves open or close, and a graphical user interface, written in MATLAB, to provide instructions and aid in automation of experimental workflow. The microfluidic chip is placed in a stage-top incubator and the cells, on-chip, are imaged using fluorescence microscope. B Two-layer microfluidic chip design where flow in sixteen channels (blue) is controlled using eight control lines (red). The inlet channels on the chip are controlled by additional control lines (labeled 9–16) and the outlet is controlled by control port 17. Each flow channel is integrated with cell isolation unit, in green, that has pillar-like traps to physically isolate single cells upon contact. C The pillars, fabricated from PDMS, are separated by a small gap of 4 µm and incorporates a V-shaped cup to efficiently hold individual cells in place. D The control lines, in the top layer, upon orthogonally intersecting the flow lines, in the bottom layer creates several thin pneumatic push-down membrane valves on-chip. The pneumatic membrane valves are actuated open or close through an external pressure source that control the flow of reagents in the channels.” Reproduced under Creative Commons Attribution 4.0 International License from Sinha, N., Yang, H., Janse, D. et al. Microfluidic chip for precise trapping of single cells and temporal analysis of signaling dynamics. Commun Eng 1, 18 (2022).
Figures and the abstract are reproduced from Sinha, N., Yang, H., Janse, D. et al. Microfluidic chip for precise trapping of single cells and temporal analysis of signaling dynamics. Commun Eng 1, 18 (2022). https://doi.org/10.1038/s44172-022-00019-2
Read the original article: Microfluidic chip for precise trapping of single cells and temporal analysis of signaling dynamics


