08 May Microfluidic platform for screening cardiac disease biomarkers
Rapid and in-time monitoring of disease-specific biomarkers holds a significant value in disease prevention and early treatment. It also is important to monitor these biomarkers post-treatment to make sure of the efficiency of the treatment. A multi-disciplinary team of researchers has recently reported a microfluidic device for multiplex biomarker screening. This platform is called PRADA (portable reusable accurate diagnostics with nanostar antennas) and the result of this study is published in bioengineering and translational medicine journal.
Currently, the gold-standard methods for detection and measurement of the biomarkers in clinical samples are enzyme-linked immunosorbent assays (ELISA) and mass spectrometry. However, these methods are associated with limitations such as low-throughput, high cost, and complex operations. Therefore, accurate diagnostic platforms with low cost and simple operations can significantly simplify the disease-specific biomarker screening process and make these tests more accessible.
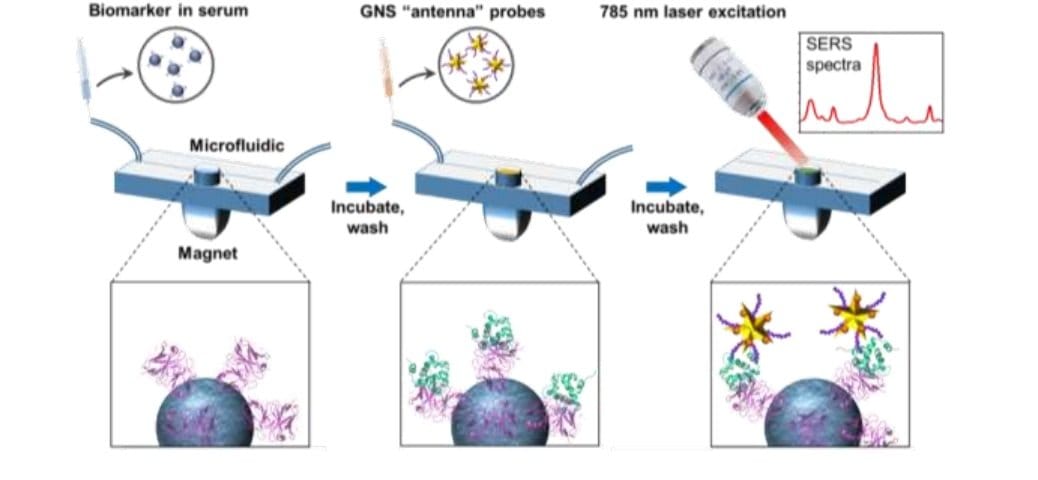
The proposed microfluidic chip includes a microchannel in which a sandwich immunoassay is assembled. For the immunoassay, first magnetic beads functionalized with antibodies formed a monolayer in the device. Then, the samples were introduced and time was given so the biomarkers attach to the antibodies. Next, gold nanostar labeled with Raman tags were introduced to bind to the biomarkers and be used for readout. The GNS barcodes bound to various points on the biomarkers. The measurements were done with a Raman setup that employed a 787 nm laser.
“Our results showed that PRADA achieved highly sensitive detection of both biomarkers of acute myocardial infarction ideal for risk stratification. The high sensitivity and specificity of PRADA were leveraged by the peptide biorecognition elements (BREs) conjugated to GNS-SERS barcodes.”

Reproduced under creative commons attribution
The multiplex microfluidics immunoassay was conducted with two cardiac biomarkers namely cardiac troponin I (cTnI) and neuropeptide Y (NPY) which are related to cardiac arrest, ischemic stroke, myocardial damage, cardiac remodeling, and angiogenesis. The microfluidic device was shown to be successful in the multiplex capturing of these cardiac biomarkers at clinically relevant concentrations. The results revealed that the microfluidic diagnostic platform had a limit of detection of 0.0055 ng/mL for cTnI and 0.12 ng/mL for NPY.
The authors envisioned that the reported device could be used as a point of care diagnostics tool in resource-limited countries and are planning to extend their proof of concept study to be able to detect >10 biomarkers.
Read the original research article:PRADA: Portable Reusable Accurate Diagnostics with nanostar Antennas for Multiplexed Biomarker Screening

Pouriya Bayat
Pouriya is a microfluidic production engineer at uFluidix. He received his B.Sc. and M.A.Sc. both in Mechanical Engineering from Isfahan University of Technology and York University, respectively. During his master's studies, he had the chance to learn the foundations of microfluidic technology at ACUTE Lab where he focused on designing microfluidic platforms for cell washing and isolation. Upon graduation, he joined uFluidix to even further enjoy designing, manufacturing, and experimenting with microfluidic chips. In his free time, you might find him reading a psychology/philosophy/fantasy book while refilling his coffee every half an hour. Is there a must-read book in your mind, do not hesitate to hit him up with your to-read list.


