16 Jun Microfluidic meshed microwells enable label-free isolation of circulating tumor cell clusters
Abstract
“Extremely rare circulating tumor cell (CTC) clusters are both increasingly appreciated as highly metastatic precursors and virtually unexplored. Technologies are primarily designed to detect single CTCs and often fail to account for the fragility of clusters or to leverage cluster-specific markers for higher sensitivity. Meanwhile, the few technologies targeting CTC clusters lack scalability. Here, we introduce the Cluster-Wells, which combines the speed and practicality of membrane filtration with the sensitive and deterministic screening afforded by microfluidic chips. The >100,000 microwells in the Cluster-Wells physically arrest CTC clusters in unprocessed whole blood, gently isolating virtually all clusters at a throughput of >25 mL/h, and allow viable clusters to be retrieved from the device. Using the Cluster-Wells, we isolated CTC clusters ranging from 2 to 100+ cells from prostate and ovarian cancer patients and analyzed a subset using RNA sequencing. Routine isolation of CTC clusters will democratize research on their utility in managing cancer.”

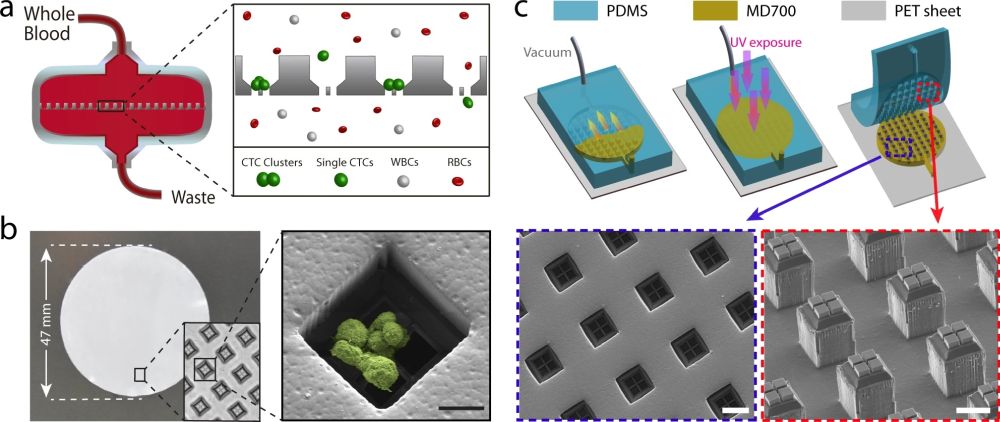
“a Schematic illustration of the Cluster-Wells’ working principle. While single cells pass unimpeded, the Cluster-Wells captures CTC clusters owing to their multicellular morphology from blood samples of cancer patients, independent of cancer type and their molecular character. b A photo of the Cluster-Wells manufactured in the form of a 47 mm-diameter membrane to be used in commercial filter holder. Close-up image (inset) shows individual wells designed to capture CTC clusters. (Right) Scanning electron micrograph of a blood-spiked LNCaP cluster as captured by one of the wells on the device (see “Methods”: SEM sample preparation and imaging). Scale bar, 20 μm. c Schematic illustration of the fabrication process developed to mold polymer Cluster-Wells device. Steps shown in the figure exclude the fabrication process for the silicon mold and the construction of reusable PDMS mold, which can be found in Supplementary Figs. 2 and 3. Scanning electron micrographs of the PDMS structure employed for micromolding (lower right) and the finished device fabricated out of photocurable polymer, MD700 (lower left). Scale bars, 50 μm.” Reproduced under Creative Commons Attribution 4.0 International License from Boya, M., Ozkaya-Ahmadov, T., Swain, B.E. et al. High throughput, label-free isolation of circulating tumor cell clusters in meshed microwells. Nat Commun 13, 3385 (2022).
Figures and the abstract are reproduced from Boya, M., Ozkaya-Ahmadov, T., Swain, B.E. et al. High throughput, label-free isolation of circulating tumor cell clusters in meshed microwells. Nat Commun 13, 3385 (2022). https://doi.org/10.1038/s41467-022-31009-9 under Creative Commons Attribution 4.0 International License.
Read the original article: High throughput, label-free isolation of circulating tumor cell clusters in meshed microwells


