06 Aug Microfluidic kidney chip for detection of drug-induced toxicity
A major challenge in drug development pipeline lies in avoiding organ-specific toxicity. Microfluidic in vitro models for drug toxicity screening such as organ on chips can play an important role in this sense by providing robust methods for evaluating cellular responses in a controllable microenvironment. For this week’s research highlight, we have selected a microfluidic kidney model reported in Scientific Reports that explores a commonly reported drug-induced complication in the proximal tubule (PT) by taking advantage of integrated sensors for measuring transepithelial electrical resistance (TEER).
“In this paper, we explore the ability to detect drug-induced kidney toxicity in primary PT cells using TEER sensing in a high-throughput model, while screening the effects of two flow conditions and microvascular co-culture. PREDICT96 is a high-throughput microfluidic system with rapid TEER measurement capability that enables parallel culture of 96 independent tissue replicates and control of multiple fluid flows within an industry-standard well-plate footprint“, the authors explained.

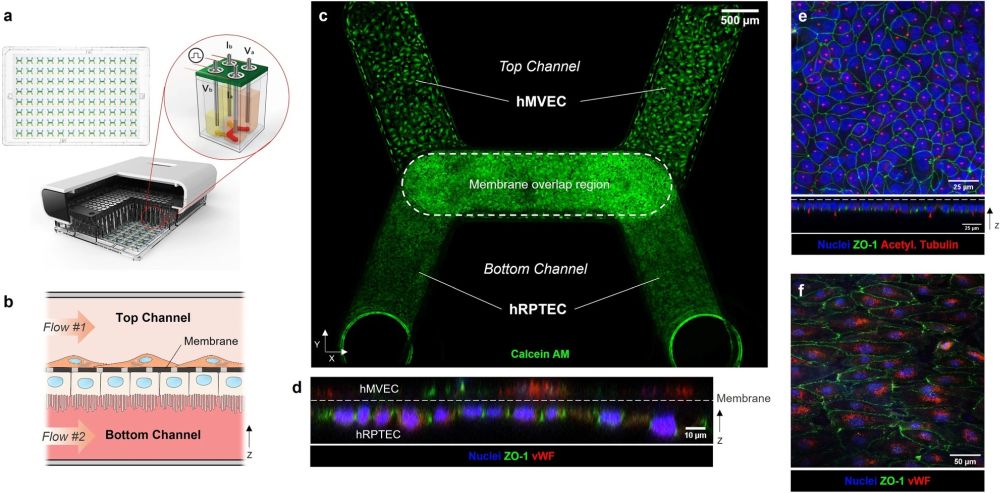
The PREDICT96 platform with integrated TEER sensing supports proximal tubule-microvascular co-culture with in-vivo-like features. (a) The PREDICT96 culture plate has 96 microfluidic devices (top left). Cross-sectional rendering of PREDICT96 Integrated TEER system (bottom) highlights the four-point TEER measurement unit (illustration, top right) in which the stainless steel pump tubes double as electrodes. (b) A cross-section schematic of the co-culture kidney model in the bilayer microfluidic device with hRPTEC on bottom of the membrane and hMVEC on top of the membrane. Fluid flow is controlled separately in top and bottom channels. (c) A confocal tile scan of a PREDICT96 device shows confluent cell layers under high FSS (0.70 dyn/cm2) on day 7 (Calcein AM, green). (d) An orthogonal view of a confocal z-stack shows hRPTEC and hMVEC on either side of the device membrane (dashed line) with hRPTEC expressing apical ZO-1 (green) and hMVEC expressing endothelial marker von Willebrand factor (vWF, red). (e, top) A maximum intensity projection of a z-stack of hRPTEC on the bottom side of the membrane shows continuous tight junctions (ZO-1, green) and abundant primary cilia (acetylated tubulin, red). (e, bottom) An orthogonal view demonstrates hRPTEC apical expression of ZO-1 and primary cilia. (f) A confocal z-slice of hMVEC on the top side of the membrane shows characteristic punctate expression of vWF (red) and tight junctions (green).” Reproduced under Creative Commons Attribution 4.0 International License from Shaughnessey, E.M., Kann, S.H., Azizgolshani, H. et al. Evaluation of rapid transepithelial electrical resistance (TEER) measurement as a metric of kidney toxicity in a high-throughput microfluidic culture system. Sci Rep 12, 13182 (2022).
“We demonstrated that TEER was an informative metric of cisplatin toxicity for hRPTEC-hMVEC co-cultures but not for hRPTEC in mono-culture. Changes in co-culture TEER correlated with hRPTEC tight junction expression and aligned with trends demonstrated by a standard cytotoxicity assay. Notably, TEER sensing revealed a decrease in co-culture barrier function due to cisplatin that occurred prior to significant increases in cell death.”, the authors explained.
Figures and the abstract are reproduced from Shaughnessey, E.M., Kann, S.H., Azizgolshani, H. et al. Evaluation of rapid transepithelial electrical resistance (TEER) measurement as a metric of kidney toxicity in a high-throughput microfluidic culture system. Sci Rep 12, 13182 (2022). https://doi.org/10.1038/s41598-022-16590-9
Read the original article: Evaluation of rapid transepithelial electrical resistance (TEER) measurement as a metric of kidney toxicity in a high-throughput microfluidic culture system


