29 Jul Microfluidic chips help with oocyte analysis and cryopreservation
The majority of microfluidic devices for Assisted Reproductive Technologies (ART) have aimed at enhancing sperm selection and analysis so far. A novel microfluidic chip, however, turns the page by offering a more efficient protocol for oocyte cryopreservation. The design rationale and performance of this microfluidic device that is developed by researchers at the University of Science and Technology of China can be found in the Nature Microsystems & Nanoengineering journal.
Oocyte cryopreservation
Oocytes (immature eggs) are often cryopreserved to delay the pregnancy due to a variety of reasons such as cancer treatment. Additionally, preserving the oocytes can enable pregnancy at older ages since most ageing-related infertilities are due to germ cell deterioration while the uterus is often still functional. Cryopreserving the oocytes, however, can be challenging. The permeability of the oocyte to cryoprotectants and osmotic transport of these chemicals into the cell can induce damage. This new microfluidics technology aims at measuring the permeability of the oocyte membrane to water (Lp) and small molecules (Ps).
“Accurate characterization of the permeability of the oocyte membrane is of great significance to the investigation of oocyte cryopreservation, assisted reproduction, and reproductive pharmacology. Here, a specific microfluidic chip was developed to synchronously and nondestructively measure the permeability of multiple mouse oocytes.”, the authors explained.
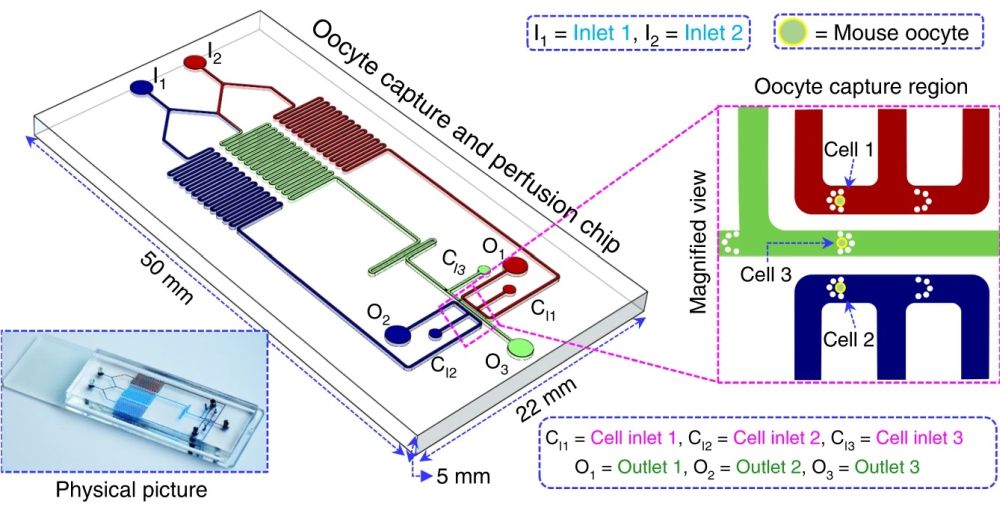
Design of the microfluidic device and working principle
The microfluidic chip is made of polydimethylsiloxane (PDMS) which is a biocompatible material. It consists of three separate microchannels with similar functionalities for the simultaneous analysis of multiple oocytes. The microchannels (200 μm wide and 150 μm high) are connected to two inlets for perfusing the oocytes with different chemical concentrations (I1 and I2) and three other inlets to introduce the oocytes to the chip (C1, C2, and C3). The cell inlets are connected to three microchannels in which a set of pillars is placed that serve as the traps for the cells. Upon trapping, the cells get exposed to three different concentrations of the chemicals under investigation. In the middle microchannel, the contents of the I1 and I2 are mixed to perfuse the middle cell (C3) with a third concentration. The chemical concentration in the middle microchannel varies based on the flow rates and concentrations of I1 and I2. The waste is removed from the device through the three outlets (O1, O2, and O3). The cells can be imaged in real-time in the traps to observe the effect of different concentrations of the chemicals. The candidate cells with the desired performance can be retrieved from the chips for cryopreservation.

The schematic of the microfluidic setup for oocyte permeability analysis. Reproduced under Creative Commons Attribution 4.0 International License.
Microfluidic analysis of mice oocyte permeability
The research team first conjured up a machine-learning algorithm to train their image processing platform by employing a neural network. The algorithm was able to identify the trapped cell in the microfluidic device and monitor the changes in its size. The volume change of the oocytes can be used as a measure of their permeability to water and cryoprotectants. The microfluidic chip was tested with mice oocytes perfused with various concentrations of ethylene glycol (EG) and 1,2-propanediol (PG) and showed promising results in determining the permeability. The study indicated that “the permeability of the oocyte membrane to water and CPA both increases as the concentration increases, which is different from other cells.”

Trapping single oocyte cells in the microfluidic device. Reproduced under Creative Commons Attribution 4.0 International License.
The oocytes were retrieved after the experiments to perform in vitro fertilization (IVF) to assess the developmental potential of the oocyte postperfusion in the microfluidic device followed by embryo transfer surgery. The development rate and birth rate of the perfusion oocytes were comparable to that of the control which proved the safety of the proposed microfluidic chip with regards to the developmental capacity.
Read the original article: A microfluidic approach for synchronous and nondestructive study of the permeability of multiple oocytes

Pouriya Bayat
Pouriya is a microfluidic production engineer at uFluidix. He received his B.Sc. and M.A.Sc. both in Mechanical Engineering from Isfahan University of Technology and York University, respectively. During his master's studies, he had the chance to learn the foundations of microfluidic technology at ACUTE Lab where he focused on designing microfluidic platforms for cell washing and isolation. Upon graduation, he joined uFluidix to even further enjoy designing, manufacturing, and experimenting with microfluidic chips. In his free time, you might find him reading a psychology/philosophy/fantasy book while refilling his coffee every half an hour. Is there a must-read book in your mind, do not hesitate to hit him up with your to-read list.


