30 Jan Microengineered human organ-on-a-chip for mimicking the blood-brain barrier
The blood-brain barrier (BBB) is a mechanism in the periphery of the central nervous system that protects the nervous system from external substances as well as hormones and neurotransmitters released in other parts of the body. This highly selective and semi-permeable membrane is essential in helping the brain maintain the homeostasis. Dysfunction or malfunction of BBB can be very catastrophic and is believed to be related to neurodegenerative diseases such as multiple sclerosis.
The blood-brain barrier consists of a tight 3D network of endothelial cells, astrocytes, and pericytes. Although the presence of BBB is crucial for the normal behavior of the brain, it also imposes challenges for delivering drugs to the central nervous system. Normally, the blood-brain barrier resists transferring external substances to the brain. It includes drug compounds as well as nano-vesicles carrying medicine. This would particularly affect the development of novel drugs for brain treatment. In vivo and in vitro models can be helpful in this regard. However, animal models are not effective and cannot necessarily be extrapolated to humans. In vitro models are also not sophisticated enough to model the complexity of the BBB. An in vitro model that is capable of modeling the complexity and multilayered structure of the BBB can have a significant effect on this research field and pave the way for novel and more effective brain treatment drugs. Microfluidics has been shown to be able to address these challenges to a great extent.
Lately, a research group has proposed a microfluidic device lined with endothelial cells, pericytes, and astrocytic networks for recapitulating the BBB. The reported microengineered physiological system (MPS) (also known as organ-on-a-chip) was tested with synthetic bioinspired nanoparticles and the results were published in Nature Communication journal in an article entitled “Microengineered human blood-brain barrier platform for understanding nanoparticle transport mechanisms”.
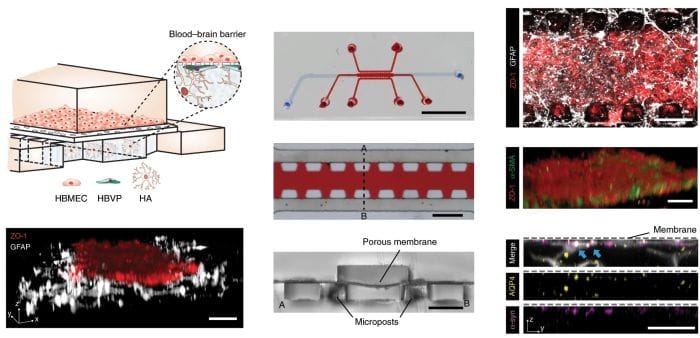
Their organ-on-a-chip device for reconstituting the blood-brain barrier structure consists of two layers separated by a 7 μm-thick porous membrane. The lower layer consists of three interconnected microchannels. The center channel houses a layer of Human brain vascular pericytes (HBVP) covered by another layer of astrocyte-laden matrigel. The two side channels fed the center channel with culture media. In the upper layer, a dense layer of human brain microvascular endothelial cells (HBMEC) were cultured. The porous membrane between the upper and lower layers allowed intercellular communication between the cell types. A constant flow was introduced to the upper layer to expose the endothelial cells with a shear-stress of 4 dyne/cm² corresponding to the actual shear level.

Reproduced under Creative Commons Attribution 4.0 International License
The blood-brain barrier microfluidic chip was shown to be capable of creating a tight barrier and mimicking the BBB-specific characteristic of the endothelial cells with diffusion permeability coefficients comparable to the actual brain. The porous membrane and proximity of the three cell types allowed paracrine and juxtacrine signaling between the cell types. Biological-relevancy of the proposed organ-on-a-chip was followed by testing the transport of drug compound surrogates through the BBB. For this, they synthesized bioinspired nanoparticles mimicking high-density lipoproteins (HDL) with a biologically relevant size and investigated the mechanisms and receptors involved in their transportation.
In their conclusion remark, authors mentioned: “We believe that our human BBB model could provide a widely useful tool for translational medicine research in particular for the modeling of neuroinflammation and reactive gliosis in neurological disorders”. Blood-brain barrier organ-on-a-chip devices have been showing very promising results and can be used as a powerful substitute for current in vitro and animal models to predict pharmacokinetics and efficiency of new brain-targeting medicines.
Read the original research article: Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms

Pouriya Bayat
Pouriya is a microfluidic production engineer at uFluidix. He received his B.Sc. and M.A.Sc. both in Mechanical Engineering from Isfahan University of Technology and York University, respectively. During his master's studies, he had the chance to learn the foundations of microfluidic technology at ACUTE Lab where he focused on designing microfluidic platforms for cell washing and isolation. Upon graduation, he joined uFluidix to even further enjoy designing, manufacturing, and experimenting with microfluidic chips. In his free time, you might find him reading a psychology/philosophy/fantasy book while refilling his coffee every half an hour. Is there a must-read book in your mind, do not hesitate to hit him up with your to-read list.


