17 Jun Measurement of single-cell metabolism using a microfluidic platform
It was only less than 100 years ago that Sir Hans Adolf Krebs measured the metabolism rate of muscle cells by exposing fresh pigeon breast meat to different reagents to discover the Krebs cycle (also known as citric acid cycle or tricarboxylic acid cycle or TCA cycle). He measured the bulk metabolism of the muscle tissue by employing a bulky manometer to measure the partial pressure of oxygen which in turn was associated with the metabolism. The idea of high throughput and accurate single-cell metabolism measurement could have been not much more than a fantasy.
Microfluidics has realized the lifelong wish of many scientists by enabling single-cell analysis at high throughput and accuracy. A novel microfluidic chip has even further expanded the capabilities of microfluidic technology at the single-cell level by offering an accurate method for single-cell calorimetry. In a recent study published in Nature Communications, a group of researchers from the University of California San Diego explained their microfluidic fabrication technique for single-cell calorimetry.
“We demonstrate on-chip single-cell calorimetry measurement with metabolic heat rates ranging from 1 to 4 nW, which are found to correlate well with the cell size.”, the authors explained.
Reproduced under Creative Commons Attribution 4.0 International License.
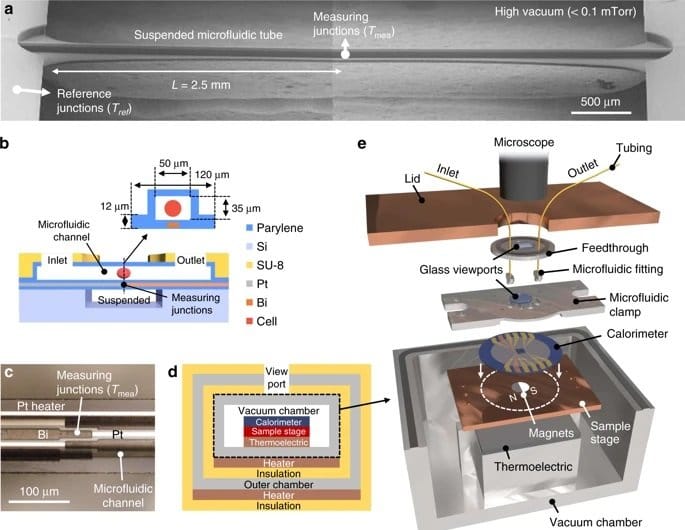
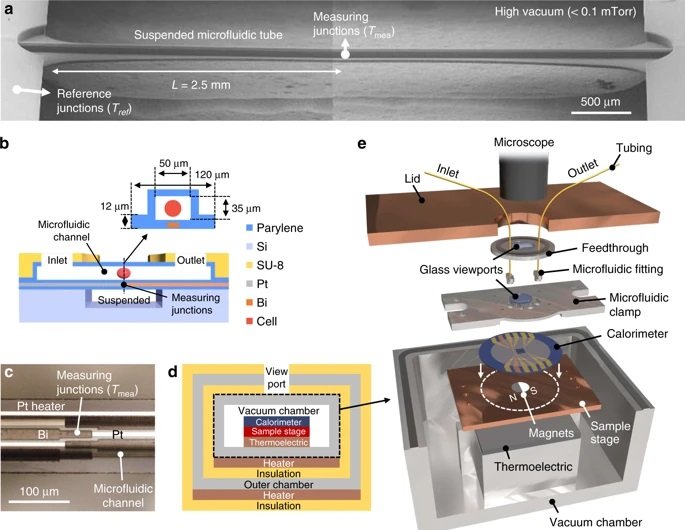
The microfluidic device consisted of a one-dimensional microchannel with extensive thermal insulation for high accuracy. Tetrahymena cells that are often used as a mammalian cell model were used as a proof of concept. A major challenge in some cell types such as Tetrahymena is that they can move in pursuit of nutrients using cilia. In this case, the cells were fed with Iron oxide and then fixed atop of a thermopile in the microchannel using an external magnetic field. The microfluidic channels allowed nutrients and oxygen to be delivered to the cell to ensure the cells’ regular activity during the experiments. However, the flow was not too harsh to overcome the magnetic field and displace the cell. Next, a thermopile, which converts thermal energy to electrical energy, underneath the microchannel recorded the thermal activity with 0.2 nW accuracy. Finally, the cells were removed from the microfluidic device by applying a more rigorous flow to remove the cells and record the baseline signal. The chip was designed such that the cell could get exposed to various reagents such as mitochondrial uncoupling agent FCCP to investigate their effect on cell metabolism.
The proposed microfluidic platform for single-cell calorimetry was shown to be promising and an order of magnitude higher in precision compared to the currently available techniques. The authors envisioned their microfluidic device to be useful in a real-time study of single-cell events such as proliferation and embryonic development.
Read the original article: Sub-nanowatt microfluidic single-cell calorimetry

Pouriya Bayat
Pouriya is a microfluidic production engineer at uFluidix. He received his B.Sc. and M.A.Sc. both in Mechanical Engineering from Isfahan University of Technology and York University, respectively. During his master's studies, he had the chance to learn the foundations of microfluidic technology at ACUTE Lab where he focused on designing microfluidic platforms for cell washing and isolation. Upon graduation, he joined uFluidix to even further enjoy designing, manufacturing, and experimenting with microfluidic chips. In his free time, you might find him reading a psychology/philosophy/fantasy book while refilling his coffee every half an hour. Is there a must-read book in your mind, do not hesitate to hit him up with your to-read list.


