18 Aug Hydrogel beads as solid-phase protein displays for functional assays and protein engineering
Abstract
“The assembly of robust, modular biological components into complex functional systems is central to synthetic biology. Here, we apply modular “plug and play” design principles to a solid-phase protein display system that facilitates protein purification and functional assays. Specifically, we capture proteins on polyacrylamide hydrogel display beads (PHD beads) made in microfluidic droplet generators. These monodisperse PHD beads are decorated with predefined amounts of anchors, methacrylate-PEG-benzylguanine (BG) and methacrylate-PEG-chloroalkane (CA), that react covalently with SNAP-/Halo-tag fusion proteins, respectively, in a specific, orthogonal, and stable fashion. Anchors, and thus proteins, are distributed throughout the entire bead volume, allowing attachment of ∼109 protein molecules per bead (⌀ 20 μm) ─a higher density than achievable with commercial surface-modified beads. We showcase a diverse array of protein modules that enable the secondary capture of proteins, either noncovalently (IgG and SUMO-tag) or covalently (SpyCatcher, SpyTag, SnpCatcher, and SnpTag), in mono- and multivalent display formats. Solid-phase protein binding and enzymatic assays are carried out, and incorporating the photocleavable protein PhoCl enables the controlled release of modules via visible-light irradiation for functional assays in solution. We utilize photocleavage for valency engineering of an anti-TRAIL-R1 scFv, enhancing its apoptosis-inducing potency ∼50-fold through pentamerization.”

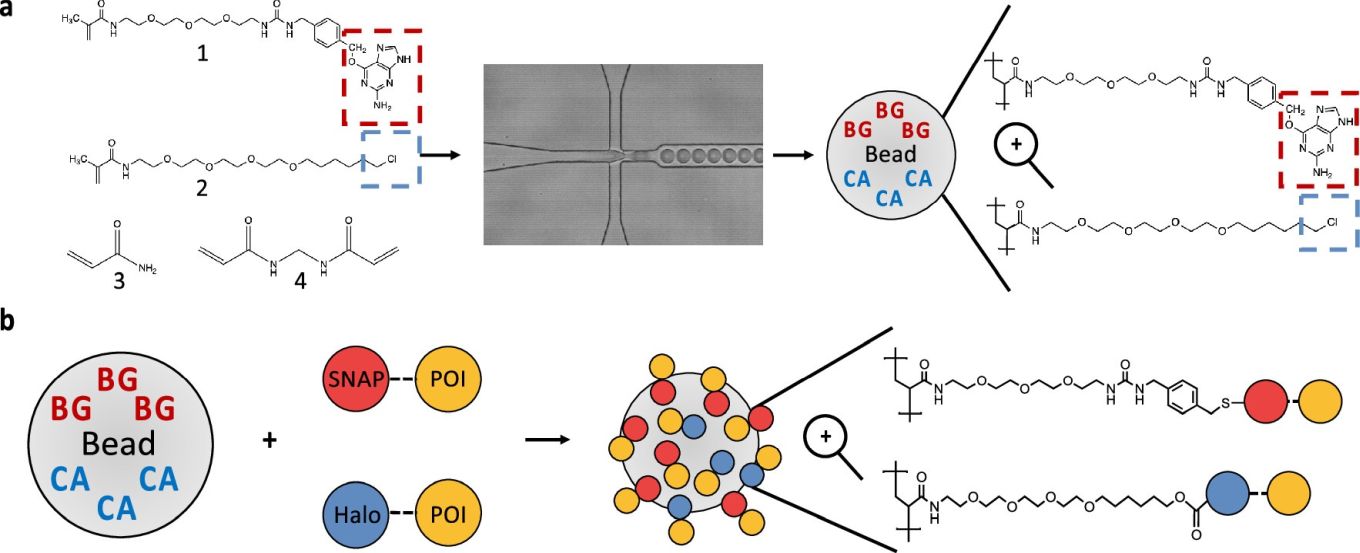
Modular polyacrylamide hydrogel display. (a) Monodisperse polyacrylamide hydrogel beads are made through the encapsulation of monomers [(1) methacrylate-PEG-benzylguanine (BG), (2) methacrylate-PEG-chloroalkane (CA), (3) acrylamide, (4) bis-acrylamide] with polymerization-inducing catalysts using droplet-based microfluidics. Upon de-emulsification, BG (red) and/or CA (blue) are retained within each bead due to copolymerization with the hydrogel backbone. (b) Hydrogel beads can then be orthogonally functionalized with SNAP- or Halo-tag fusion proteins (red and blue, respectively) through covalent reaction with their respective copolymerized small molecule ligands (BG/CA).” Reproduced under Creative Commons Attribution 4.0 International License from Gigavalent Display of Proteins on Monodisperse Polyacrylamide Hydrogels as a Versatile Modular Platform for Functional Assays and Protein Engineering Thomas Fryer, Joel David Rogers, Christopher Mellor, Timo N. Kohler, Ralph Minter, and Florian Hollfelder ACS Central Science Article ASAP.
Figures and the abstract are reproduced from Gigavalent Display of Proteins on Monodisperse Polyacrylamide Hydrogels as a Versatile Modular Platform for Functional Assays and Protein Engineering Thomas Fryer, Joel David Rogers, Christopher Mellor, Timo N. Kohler, Ralph Minter, and Florian Hollfelder ACS Central Science Article ASAP DOI: 10.1021/acscentsci.2c00576
Read the original article: Gigavalent Display of Proteins on Monodisperse Polyacrylamide Hydrogels as a Versatile Modular Platform for Functional Assays and Protein Engineering


