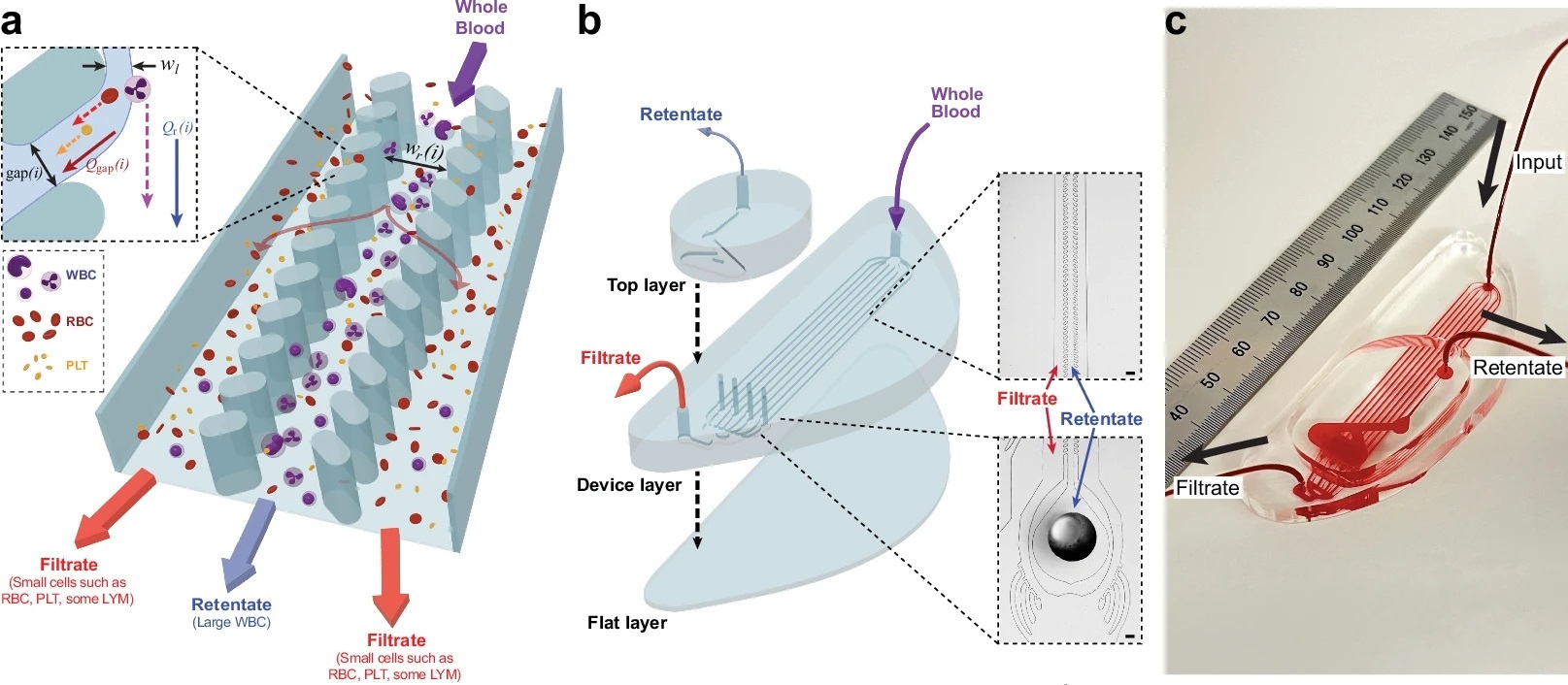
03 Mar Exploring Microfluidic Leukapheresis for Pediatric Leukemia
Leukapheresis is a critical treatment for children with symptomatic hyperleukocytosis, a condition where extremely high white blood cell (WBC) counts can lead to severe complications such as tumor lysis syndrome, blood clotting disorders, and organ damage. However, the conventional centrifugation-based leukapheresis technique has significant drawbacks, particularly for pediatric patients. The large extracorporeal blood volume required (hundreds of milliliters), high flow rates, and substantial platelet loss pose serious risks, including low blood pressure, anemia, and the need for invasive central venous access.
This study explores whether a microfluidic-based leukapheresis system can provide a safer, more effective alternative by reducing the extracorporeal blood volume while maintaining efficient WBC removal. The researchers developed and tested a high-throughput microfluidic leukapheresis system that selectively removes large WBCs while minimizing platelet and red blood cell (RBC) loss. Unlike traditional systems, this low-volume extracorporeal circuit reduces the strain on pediatric patients and eliminates the need for RBC priming. Their microfluidic chip is based on controlled incremental filtration (CIF), a method that efficiently separates WBCs from whole blood using microfluidic channels instead of centrifugation.
“Here, we tested whether performing cell separation with a high-throughput microfluidic technology could alleviate these limitations. In vitro, our microfluidic devices removed ~85% of large leukocytes and ~90% of spiked leukemic blasts from undiluted human whole blood, while minimizing platelet losses.“, the authors explained.
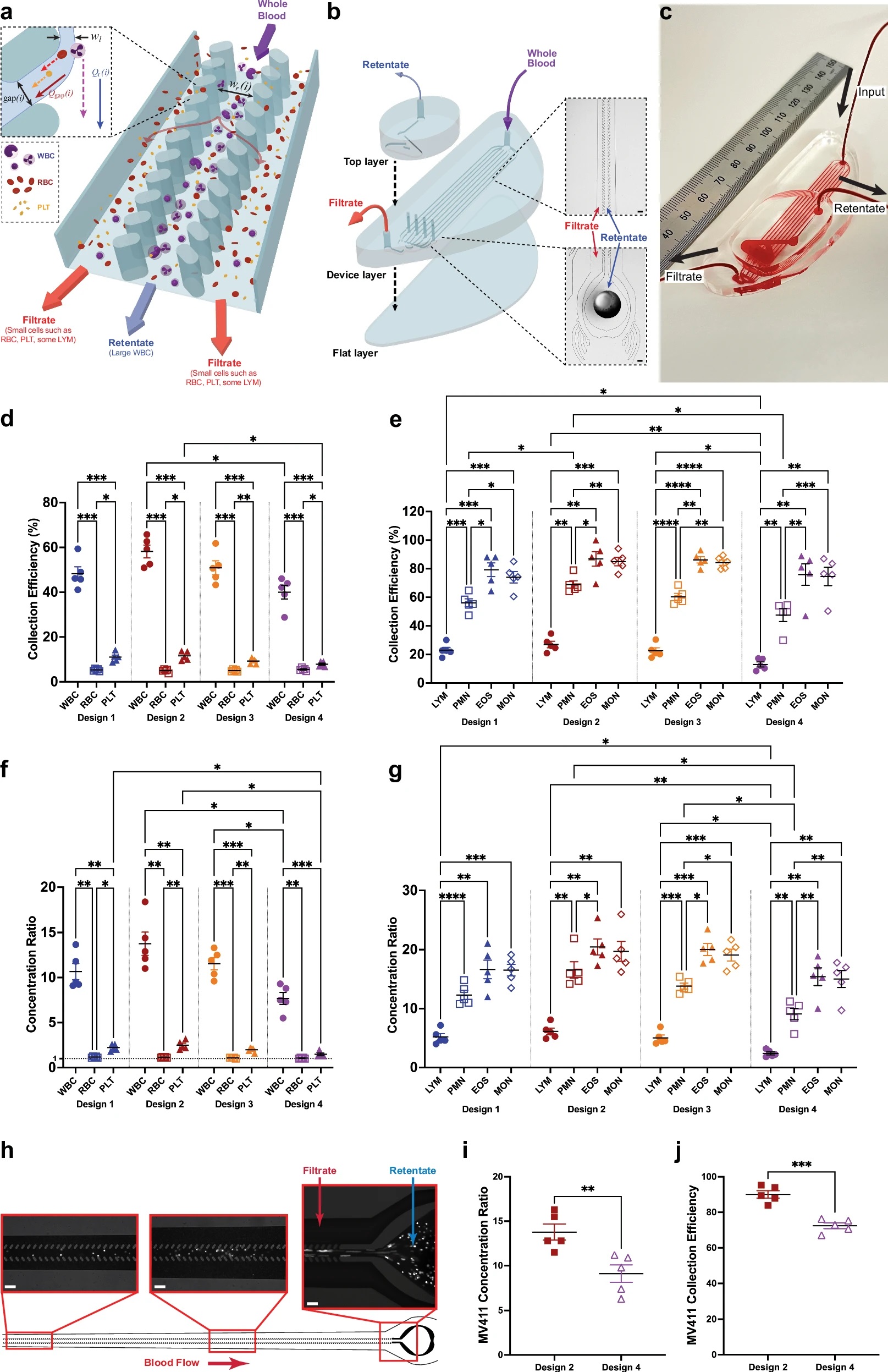
“a Schematic showing how the CIF channels operate. As whole blood enters a CIF element, smaller cells (e.g., RBC and PLT) exit through the gaps i, while larger cells (e.g., WBC) remain in the central retentate channel. b Schematic showing the assembly of an 8-element CIF device. As whole blood enters the input port of the device layer (top right), the blood is separated into 8 parallel CIF elements. Each element’s filtrate is merged and exits from the filtrate output port. WBC in the retentate exit vertically into a collecting top layer and are removed from the retentate output port. c Photograph of a blood-filled CIF device (ruler shown in mm). d, e Collection efficiency data highlights CIF’s ability to separate large cells. RBC and PLT loss are kept to <15%, while large WBCs such as MON and EOS are collected up to an efficiency of ~85%. f The concentration ratio of all designs was significantly higher for WBC than RBC and PLT, with the RBC concentration ratio being close to 1 across all designs. g Among WBC subtypes, EOS and MON exhibited the highest concentration ratios across all designs, followed by PMN. LYM, due to their small size, were least concentrated. h Images of fluorescently labeled MV-4−11 cells spiked into whole blood flowing within the device. Note how most leukemic cells are located within the retentate channel with little to none in the filtrate channels. i, j Both designs were able to effectively concentrate (i, p = 0.0056) and remove (j, p = 0.0007) MV-4−11 leukemic cells from whole blood, with Design 2 being more efficient than Design 4. Data shown as mean ± s.e.m. n = 5 for both healthy and MV-4−11 spiked blood data. Scale bar = 100 µm; wl=width of the fluid lamina, Qr(i)=retentate channel flow, Qgap(i)=filtration gap flow. Data analyzed by (d–g) 2-way RM ANOVA with Tukey’s multiple comparison test or (i, j) 2-tailed paired t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.” Reproduced from Iqbal, M., McLennan, A.L., Mukhamedshin, A. et al. Ultra-low extracorporeal volume microfluidic leukapheresis is safe and effective in a rat model. Nat Commun 16, 1930 (2025). under a CC BY 4.0 Attribution 4.0 International license
The microfluidic device was tested with undiluted human blood, achieving ~85% removal of large WBCs and ~90% removal of leukemic blasts while keeping RBC and platelet loss below 10%. The device was scaled up by multiplexing several microfluidic units, allowing it to handle clinically relevant flow rates. To evaluate the system in a living model, researchers conducted 3-hour leukapheresis procedures on anesthetized rats, comparing microfluidic leukapheresis with a sham control. The microfluidic device operated at a very low extracorporeal volume (~2.9% of the rat’s total blood volume), demonstrating its feasibility for small patients. The microfluidic chip successfully removed ~80% of target WBCs while maintaining stable blood pressure and normal organ function.
The microfabricated microfluidic platform lowered circulating WBCs by nearly 50% within 3 hours in rats, showing similar effectiveness to conventional leukapheresis. Unlike centrifugation-based systems, this approach preserved platelets and RBCs, reducing the need for blood transfusions. Plasma markers of organ function and clotting remained stable, indicating the procedure was safe. Moreover, the system can be multiplexed to achieve flow rates comparable to standard clinical leukapheresis, making it a viable alternative for pediatric use.
This study demonstrates that microfluidic leukapheresis is a safe and effective method for selectively removing WBCs from circulation with a dramatically lower extracorporeal volume than current clinical methods. The ability to operate at lower blood volumes and flow rates makes this approach especially promising for pediatric leukemia patients, reducing risks associated with conventional leukapheresis. With further refinement, this technology could offer a less invasive, safer alternative for children requiring rapid WBC reduction.
“Taken together, our data suggest that CIF-based microfluidic devices may be a feasible alternative to centrifugation-based leukapheresis in pediatric patients with acute leukemias. Ultra-low ECV, high collection efficiency for leukemic blasts, minimal PLT loss, and the ability to operate effectively at lower flow rates offered by CIF devices may obviate most of the drawbacks associated with current centrifugation-based procedure. “, the authors concluded
Figures are reproduced from Iqbal, M., McLennan, A.L., Mukhamedshin, A. et al. Ultra-low extracorporeal volume microfluidic leukapheresis is safe and effective in a rat model. Nat Commun 16, 1930 (2025). https://doi.org/10.1038/s41467-025-57003-5 under a CC BY 4.0 Attribution 4.0 International license.
Read the original article: Ultra-low extracorporeal volume microfluidic leukapheresis is safe and effective in a rat model
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


