26 Feb Exploring Dry Eye Syndrome with a Microfluidic Model
Dry Eye Syndrome (DES) presents a significant challenge in ocular health, impacting millions globally with symptoms that reduce quality of life. Traditional research methods have fallen short in replicating the complex interplay at the corneal surface under DES conditions. A novel microfluidic study involving an Organ-On-a-Chip bridges this gap, introducing an advanced Corneal Epithelium-on-Chip (CEpOC) that closely mimics the human eye’s air-liquid (AL) interface, offering fresh insights into DES and paving the way for potential treatments.
“Despite its common occurrence, there is currently no comprehensive in vitro model that accurately reproduce the cellular characteristics of DES. Here we modified a corneal epithelium-on-a-chip (CEpOC) model to recapitulate DES by subjecting HCE-T human corneal epithelial cells to an air–liquid (AL) interface stimulus. We then assessed the effects of AL stimulation both in the presence and absence of diclofenac (DCF), non-steroidal anti-inflammatory drug.“, the authors explained.
The cornerstone of this research is the microfluidic device, crafted using a blend of stereolithographic 3D printing and solution cast molding. This fabrication of microfluidic chips was conducted by producing a device from PDMS, incorporating a PET membrane to construct the microfluidic channels and chambers essential for simulating the corneal environment.
Human corneal epithelial cells (HCE-T) were cultured within this setup, aiming to emulate the corneal epithelial barrier crucial for DES studies. A specially designed medium nurtured the cells through the microfluidic chip, facilitating the development of a robust epithelial barrier over seven days.
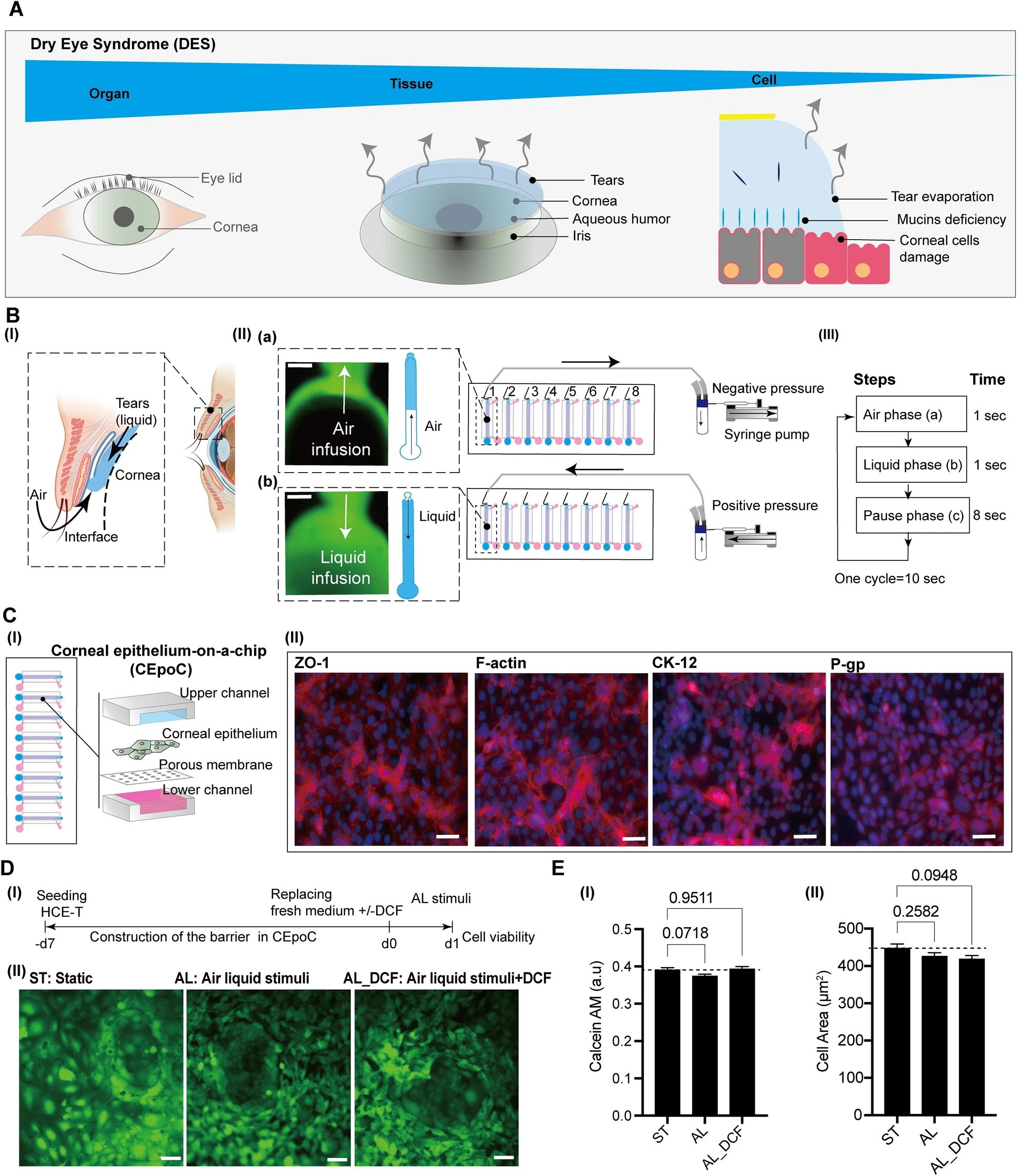
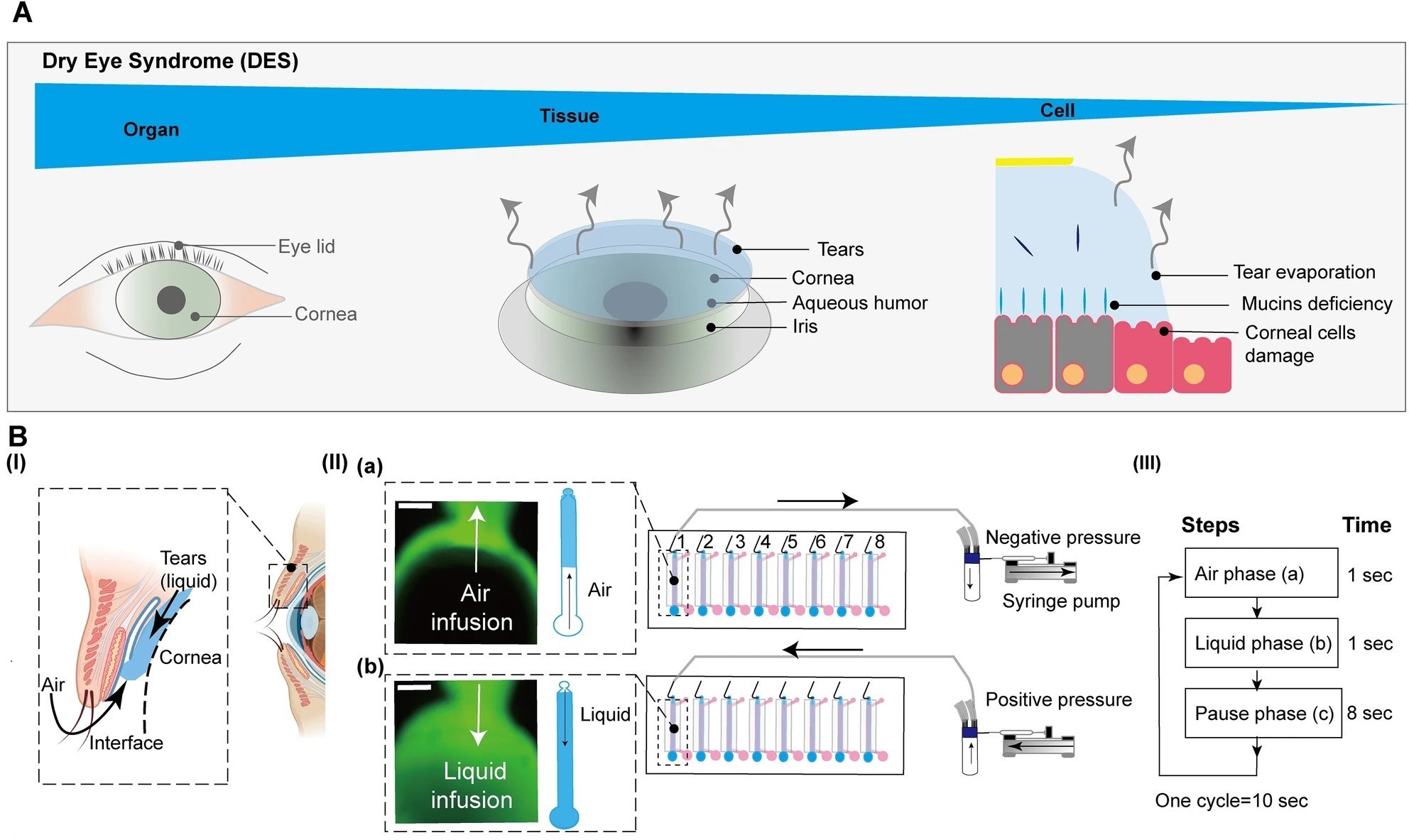
“The conceptual design and characteristics of the modified CEpOC. (A) Schematic illustration showcases the evaporative dry eye. (B) The generation of AL stimulus in the modified CEpOC. I) Schematic illustration (created with BioRender.com) showcases air–liquid interphase process; II) The application of air–liquid stimuli in the microfluidic devices showcasing air infusion (a) then liquid infusion (b); III) The steps and duration of the application of air and liquid in the microfluidic device. Green color indicates the fluorescent signal of sodium fluorescein solution in the reservoir area. Scale bar, 500 μm. (C) I) Schematic illustration showcases the microfluidic device and HCE-T culturing for forming a barrier; II) Immunofluorescence staining of ZO-1, F-actin, CK12, and P-gp. Red color indicates the fluorescent signal of the targeted protein. Blue color indicates the nucleus satin DAPI. Scale bar, 50 μm. (D) Cell viability in the microfluidic devices under ST and AL conditions with and without DCF; I) The protocol that showcase HCE-T culturing, barrier formation and the AL stimuli; II) Microscopic images of Calcein-AM under ST and AL conditions with and without DCF. (E) Microscopic signal-cell analysis of Calcein-AM intensity (I) and cell area (II). The analysis was performed on images of independent samples; 1100 cells were randomly selected and analyzed for three independent samples. Green, Calcein-AM. Scale bar, 50 μm. Data are represented in the bar plot as means ± S.E.M. The p-values were determined by the Dunnett’s multiple comparison test.” Reproduced from Kado Abdalkader, R., Chaleckis, R., Fujita, T. et al. Modeling dry eye with an air–liquid interface in corneal epithelium-on-a-chip. Sci Rep 14, 4185 (2024). under a CC BY 4.0 DEED Attribution 4.0 International license.
The study’s innovation lies in its air-liquid interface stimulus application, achieved through a meticulously designed syringe pump system. This system cyclically introduced air and liquid flow to the corneal epithelial cells, mimicking the desiccating conditions characteristic of DES. This experimental approach allowed for the observation of cellular responses under DES-like stress.
Employing a comprehensive dual-omics analysis, the study unveiled significant shifts in gene expression and metabolic profiles indicative of DES. These included enhanced inflammation and diminished mucin production, essential for maintaining ocular surface moisture.
Furthermore, the impact of Diclofenac (DCF), a commonly used anti-inflammatory drug, was scrutinized within this model. The findings offered a nuanced view of DES’s effect on corneal epithelial cells and the potential therapeutic actions of DCF. This opens new pathways for developing targeted treatments for DES, promising improved outcomes for those affected.
The Corneal Epithelium-on-Chip model exemplifies the potential of microfluidic technology in transforming biomedical research, setting the stage for innovative solutions to combat Dry Eye Syndrome and improve patient outcomes.
“Our study sheds light on the molecular and metabolic changes that underlie AL stimuli. The observed alterations suggest a cascade of events involving inflammation, disrupted cellular structure, and altered metabolism. Importantly, our findings raise questions about the effectiveness of DCF in mitigating these effects. “, the authors concluded.
Figures are reproduced from Kado Abdalkader, R., Chaleckis, R., Fujita, T. et al. Modeling dry eye with an air–liquid interface in corneal epithelium-on-a-chip. Sci Rep 14, 4185 (2024). https://doi.org/10.1038/s41598-024-54736-z under a CC BY 4.0 DEED Attribution 4.0 International license.
Read the original article: Modeling dry eye with an air–liquid interface in corneal epithelium-on-a-chip
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


