24 Jan Droplet microfluidic-assisted bioprinting of functional living materials
Abstract
“Living materials bring together material science and biology to allow the engineering and augmenting of living systems with novel functionalities. Bioprinting promises accurate control over the formation of such complex materials through programmable deposition of cells in soft materials, but current approaches had limited success in fine-tuning cell microenvironments while generating robust macroscopic morphologies. Here, we address this challenge through the use of core-shell microgel ink to decouple cell microenvironments from the structural shell for further processing. Cells are microfluidically immobilized in the viscous core that can promote the formation of both microbial populations and mammalian cellular spheroids, followed by interparticle annealing to give covalently stabilized functional scaffolds with controlled microporosity. The results show that the core-shell strategy mitigates cell leakage while affording a favorable environment for cell culture. Furthermore, we demonstrate that different microbial consortia can be printed into scaffolds for a range of applications. By compartmentalizing microbial consortia in separate microgels, the collective bioprocessing capability of the scaffold is significantly enhanced, shedding light on strategies to augment living materials with bioprocessing capabilities.”
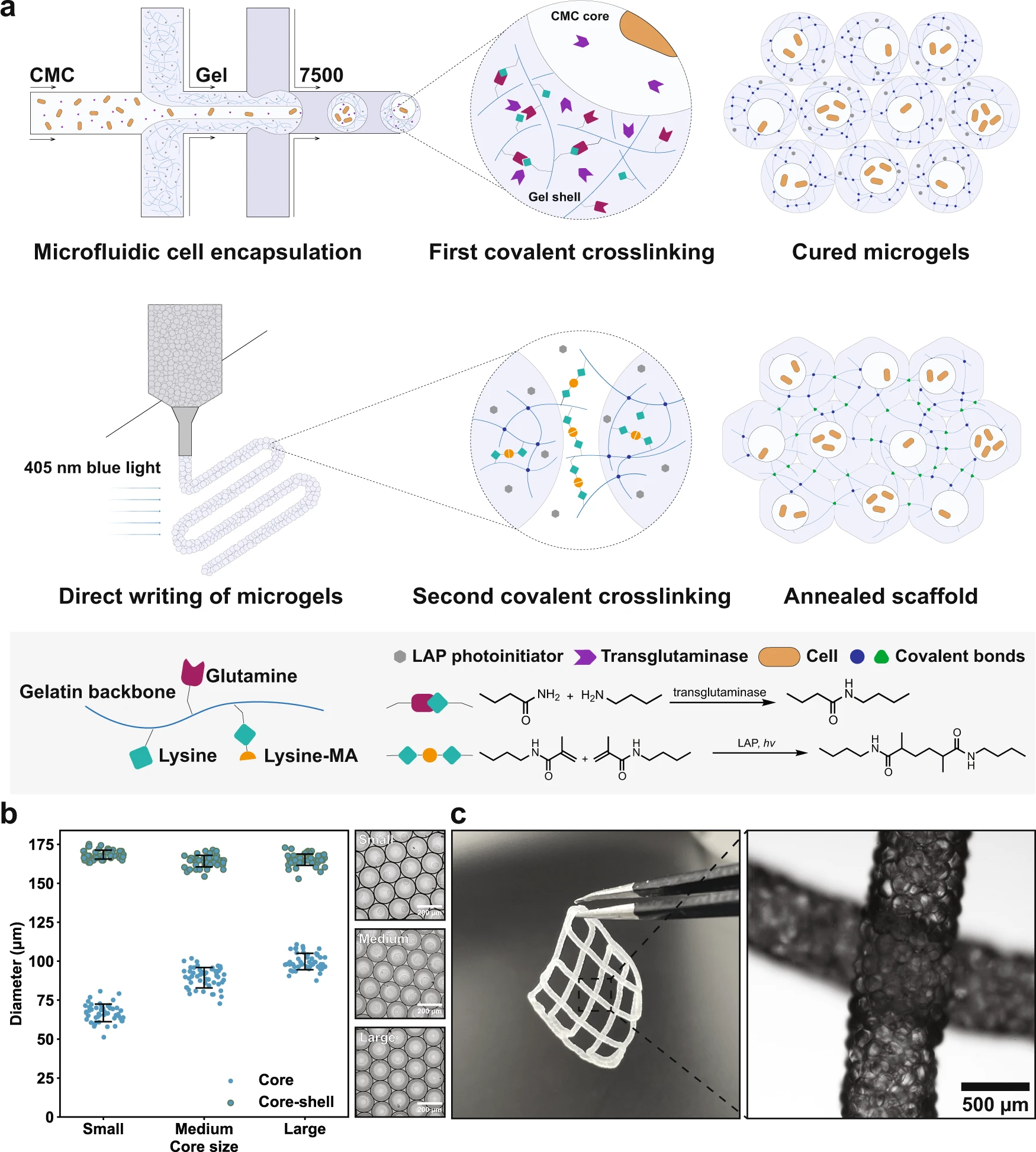
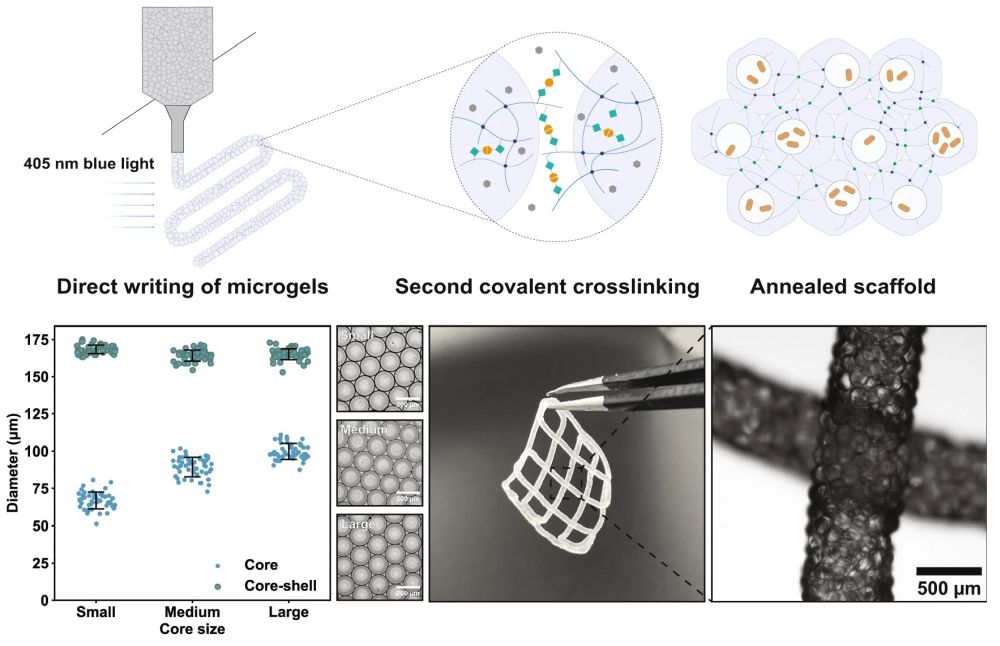
“a Schematic of the formation of PAM scaffolds. First, the LAP-containing gelatin/gelMA polymer blend, together with cell-containing CMC solution spiked with transglutaminases, is emulsified by carrier oil into droplets within a flow-focusing microfluidic device. Droplets are then converted into microgels by enzymatic reactions, after which they are extruded and patterned into scaffolds. Finally, blue light initiates a second covalent crosslinking among jammed microgels, giving rise to PAM scaffolds. b By microfluidics, the core-shell ratio can be controlled. Flow rates of the hydrogel precursor solution (shell phase) and the carrier oil are controlled at 8 and 40 µL/min, respectively, and the flow rates of CMC (core phase) are 1, 2, and 3 µL/min, for small, medium, and large core size, respectively, n = 50 microgels analyzed in one experiment. c Printed PAM scaffolds and the micrograph shows local magnification of two intersected filaments. Data are presented as mean values ± standard deviation and source data are provided as a Source Data file.” Reproduced under a Creative Commons Attribution 4.0 International License from Ou, Y., Cao, S., Zhang, Y. et al. Bioprinting microporous functional living materials from protein-based core-shell microgels. Nat Commun 14, 322 (2023).
Figures and the abstract are reproduced from Ou, Y., Cao, S., Zhang, Y. et al. Bioprinting microporous functional living materials from protein-based core-shell microgels. Nat Commun 14, 322 (2023). https://doi.org/10.1038/s41467-022-35140-5 under a Creative Commons Attribution 4.0 International License.
Read the original article: Bioprinting microporous functional living materials from protein-based core-shell microgels


