24 Oct Capturing the Dynamics of Extracellular Vesicles in Real-Time with Microfluidic Technology
Microfluidic technology is once again at the forefront of a significant scientific discovery, this time offering a window into the elusive world of extracellular vesicles (EVs). A recent study, detailed in Nature Communications, introduces the MelacChip – a pioneering microfluidic device that isolates and analyzes nascent EVs in real-time. This innovation promises to unlock new dimensions in our understanding of cellular communication and the role of EVs in disease.
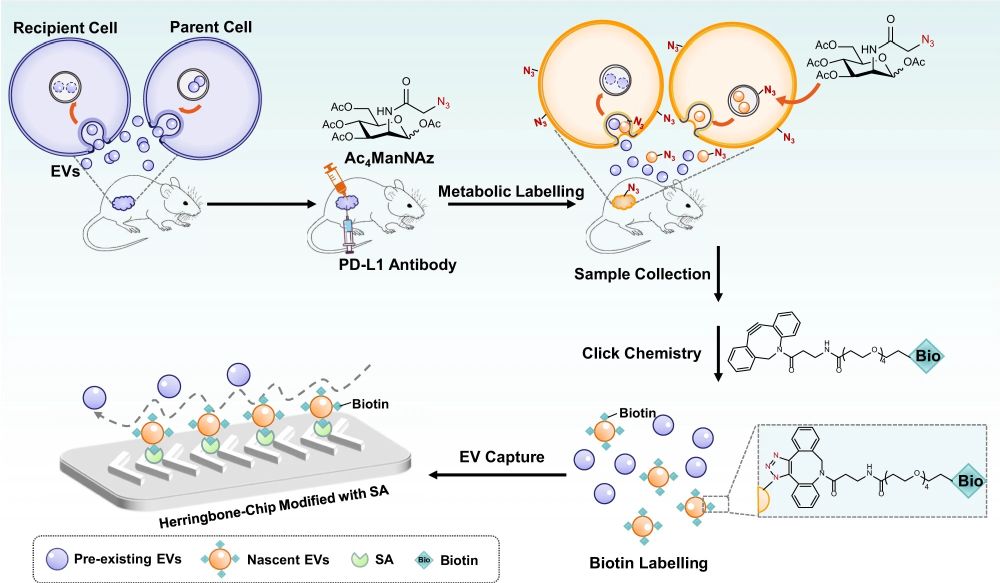
“Here, by co-translational introduction of azido groups to act as a timestamp for click chemistry labelling, we develop a microfluidic-based strategy to enable selective isolation of nascent EVs stimulated by an external cue. In two mouse models of anti-PD-L1 immunotherapy, we demonstrate the strategy’s feasibility and reveal the high positive correlation of nascent PD-L1+ EV level to tumor volume, suggesting an important role of nascent EVs in response to immunotherapy in cancer treatment.“, the authors explained.
Extracellular vesicles are nanosized entities secreted by almost all cell types. Their pivotal role in mediating cell-cell communication has been linked to various physiological and pathological processes, making them potential biomarkers for a plethora of diseases, including cancer and neurodegenerative disorders. However, the challenge of temporally sorting EVs—distinguishing newly produced ones from preexisting—has been a significant hurdle in the field.
Leveraging the precision and efficiency of microfluidic devices, researchers have developed a strategy to overcome this challenge. By introducing azido groups cotranslationally, acting as a timestamp for click chemistry labelling, the team engineered a microfluidic-based approach to selectively isolate nascent EVs stimulated by an external cue. This innovation is encapsulated in the MelacChip, a microfluidic chip that combines metabolic glycan labeling and click chemistry for the efficient and selective capture of nascent EVs.
The Microfluidic Investigation
In the study, two mouse models of anti-PDL1 immunotherapy were employed to demonstrate the strategy’s feasibility. The mice were treated with unnatural sugars for in vivo metabolic glycoengineering, ubiquitously labeling the newly-produced EVs with azido groups. This labeling acted as a timestamp, distinguishing them from preexisting populations. Subsequent click chemistry linked the azido-labeled EVs with alkynyl biotin, enabling their specific capture by a streptavidin-modified herringbone microfluidic chip.
The herringbone chip, known for generating steady chaotic flows, enhanced the collisions between EVs and the affinity interface, ensuring efficient capture. This microfluidic innovation allowed the researchers to quantify the relative amount of EV produced after each immunotherapy session, offering real-time insights into the dynamic world of extracellular vesicles

“Tumor-bearing mice were simultaneously treated with PD-L1 antibody and Ac4ManNAz (tetraacetylated N-Azidoacetyl-mannosamine), an unnatural sugar for metabolic labeling of EVs with azido groups. Subsequently, the azido-labeled EVs were tagged with biotin groups by click chemistry and captured by streptavidin (SA)-modified herringbone microfluidic chip.” Reproduced from Wu, Q., Wang, W., Zhang, C. et al. Capturing nascent extracellular vesicles by metabolic glycan labeling-assisted microfluidics. Nat Commun 14, 6541 (2023). under Creative Commons Attribution 4.0 International License.
The MelacChip strategy was validated through a series of rigorous tests. Dynamic light scattering was employed to analyze the properties of labeled EVs, confirming their similarity to non-labeled EVs. The labeled EVs exhibited high expression of CD63, a classical pan-EV marker. Proteomics study further corroborated the biocompatibility of the metabolic glycan labeling, revealing a high correlation among biological replicates and confirming that the additive affected neither EV morphology nor proteome significantly.
The integration of metabolic glycan labeling with advanced microfluidic technology heralds a new era in the study of extracellular vesicles. With the ability to capture the in vivo dynamics of EVs during immunotherapy, researchers are now equipped with a powerful tool to unravel the complex interplay of EVs in cellular communication, disease progression, and treatment response. The insights gleaned from this study promise to accelerate the discovery of EV biomarkers, opening new frontiers in diagnostics and therapeutic interventions.
“Overall, the combination of EV metabolic labeling and efficient microfluidic enrichment improve our ability to accurately analyze EV secretion over time, which should enable the study of EV secretion mechanisms as well as the in-depth exploration of EV biological function and clinical value. Besides adding a temporal dimension to our understanding of EV dynamics in immunotherapy, in principle Melac-Chip can also be applied to study EV dynamics that is stimulated by a broad range of cues, such as microbial infection, environmental (temperature, light, and gravity) variation, and diet change.“, the authors concluded.
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds.
Figures are reproduced from Wu, Q., Wang, W., Zhang, C. et al. Capturing nascent extracellular vesicles by metabolic glycan labeling-assisted microfluidics. Nat Commun 14, 6541 (2023). https://doi.org/10.1038/s41467-023-42248-9 under a Creative Commons Attribution 4.0 International License)
Read the original article: Capturing nascent extracellular vesicles by metabolic glycan labeling-assisted microfluidics


