08 Dec Anticancer precision medicine fostered by microfluidic technology
Abstract
“Precision medicine is starting to incorporate functional assays to evaluate anticancer agents on patient-isolated tissues or cells to select for the most effective. Among these new technologies, dynamic BH3 profiling (DBP) has emerged and extensively been used to predict treatment efficacy in different types of cancer. DBP uses synthetic BH3 peptides to measure early apoptotic events (‘priming’) and anticipate therapy-induced cell death leading to tumor elimination. This predictive functional assay presents multiple advantages but a critical limitation: the cell number requirement, that limits drug screening on patient samples, especially in solid tumors. To solve this problem, we developed an innovative microfluidic-based DBP (µDBP) device that overcomes tissue limitations on primary samples. We used microfluidic chips to generate a gradient of BIM BH3 peptide, compared it with the standard flow cytometry based DBP, and tested different anticancer treatments. We first examined this new technology’s predictive capacity using gastrointestinal stromal tumor (GIST) cell lines, by comparing imatinib sensitive and resistant cells, and we could detect differences in apoptotic priming and anticipate cytotoxicity. We then validated µDBP on a refractory GIST patient sample and identified that the combination of dactolisib and venetoclax increased apoptotic priming. In summary, this new technology could represent an important advance for precision medicine by providing a fast, easy-to-use and scalable microfluidic device to perform DBP in situ as a routine assay to identify the best treatment for cancer patients.”

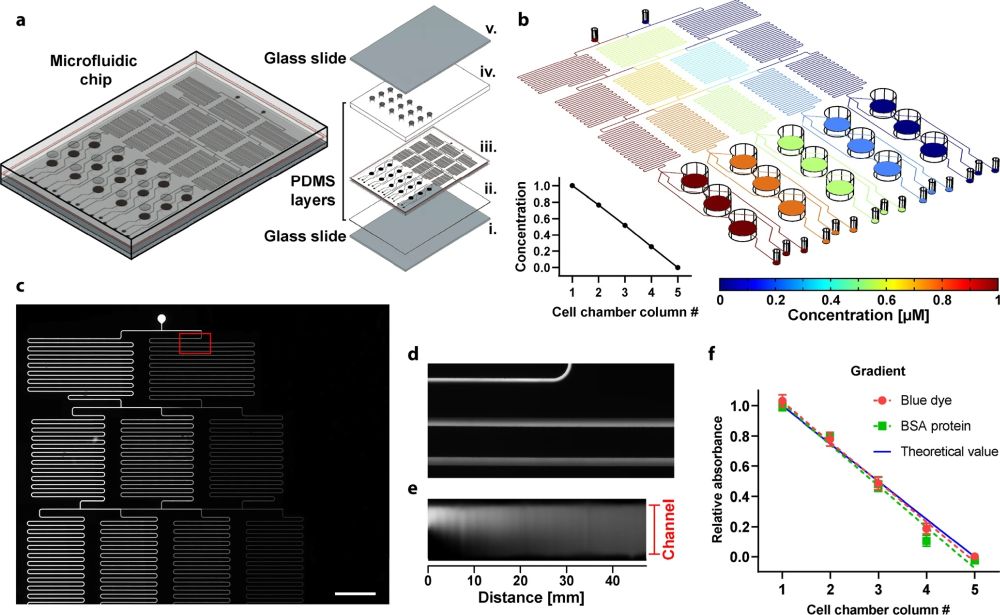
“a Schematic representation of the chip including exploded view of all the parts: (i) lower glass slide, (ii) thin PDMS layer, (iii) channels & chambers PDMS layer (1 mm thick), (iv), chambers PDMS layer (2 mm thick), and (v) upper glass slide. b Simulation of peptide concentration along with the whole microfluidic platform. The coordinate of the slice plot is selected at the middle of the microfluidic channels. Relative concentration as a function of the cell chamber (inset figure). c Fluorescence image of the network of microfluidic channels after injecting fluorescein in the left inlet and distilled water in the right inlet at a pressure of 100 mbar. d Zoomed view of the region where both solutions are mixed. e Fluorescence intensity along with the first 47 mm of the mixer channel (total channel length is ~190 mm); Note that the image was processed in ImageJ to be plotted as a straight channel f. Colorimetric detection of BSA protein and relative absorbance of blue dye at the outlets of the chip measured at 562 and 640 nm, respectively, on a plate reader. Together with the blue dye, BSA protein was injected through one of the inlets, while MiliQ water was injected in the other (inlet pressure of 200 mbar). Data represent three independent experiments using different chips.” Reproduced under a Creative Commons Attribution 4.0 International License from Manzano-Muñoz, A., Yeste, J., Ortega, M.A. et al. Microfluidic-based dynamic BH3 profiling predicts anticancer treatment efficacy. npj Precis. Onc. 6, 90 (2022).
Figures and the abstract are reproduced from Manzano-Muñoz, A., Yeste, J., Ortega, M.A. et al. Microfluidic-based dynamic BH3 profiling predicts anticancer treatment efficacy. npj Precis. Onc. 6, 90 (2022). https://doi.org/10.1038/s41698-022-00333-0 under a Creative Commons Attribution 4.0 International License.
Read the original article: Microfluidic-based dynamic BH3 profiling predicts anticancer treatment efficacy


