20 Jan Advancing Liquid Biopsies with High-Throughput Microfluidics
Circulating tumor cells (CTCs) hold the potential for cancer diagnosis and monitoring, offering a non-invasive method to track tumor evolution. However, their extreme rarity in the bloodstream and technical challenges in isolating them have severely limited their clinical utility. In a recent study published in Nature Communications, a research team developed a microfluidic chip for interrogating large blood volumes to analyze patient-derived leukapheresis products.
“To enable tumor epitope-agnostic interrogation of large blood volumes, we developed a high-throughput microfluidic device, depleting hematopoietic cells through high-flow channels and force-amplifying magnetic lenses. Here, we apply this technology to analyze patient-derived leukapheresis products, interrogating a mean blood volume of 5.83 liters from seven patients with metastatic cancer. “, the authors explained.
Researchers have developed this high-throughput microfluidic device, called the LPCTC-iChip, capable of efficiently isolating CTCs from the vast blood volumes processed during diagnostic leukapheresis. This method bypasses traditional limitations by enabling epitope-agnostic enrichment of CTCs.
The LPCTC-iChip combines two microfluidic modules: a debulking chip to remove red blood cells (RBCs), platelets, and excess plasma, and a magnetic sorter equipped with enhanced magnetic lenses for ultra-precise separation of CTCs from leukocytes. The process involves:

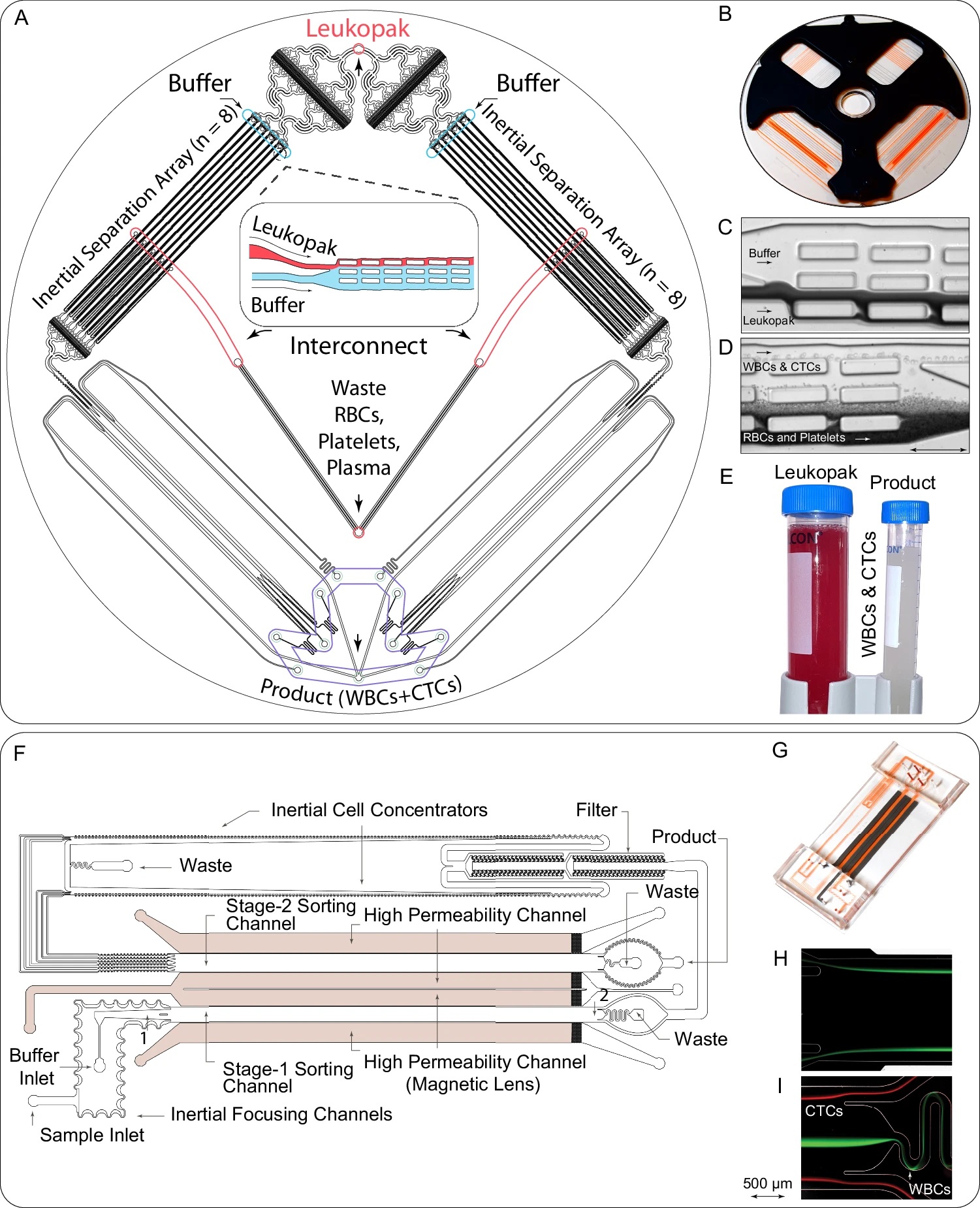
“A, B Schematic (A) and image (B) of the debulking chip used for removing unbound antibodies, RBCs, platelets, and excess plasma, concentrating WBCs and CTCs into a clean buffer. C, D Images illustrating the inertial separation of nucleated cells from RBCs and platelets within the debulking chip (n = 6). The underlying inertial separation array technology23 repeatedly deflects larger nucleated cells over an array of rectangular islands (microposts) using wall lift forces to transfer the cells into a clean buffer stream, separating them from the smaller cells and non-nucleated cells (RBCs, platelets). Following the entry into the device (C), serial deflection across the multiple islands that comprise the Chip allows the collection of nucleated cells with very high purity and yield (D). E Image of the input sample (leukopak) before debulking, with red color illustrating high RBC content, and of the purified product after debulking of RBCs and platelets (n = 6). F Design of the microfluidic magnetic sorter for depleting magnetically labeled WBCs, using cascaded two-stage magnetic sorting and a very high-gradient magnetic field, which is created by magnetic lenses adjacent to the cell flow channels22. G Image of the microfluidic magnetic sorter device. H, I Streak images of cells at the inlet (H) and exit (I) of stage I of the magnetic sorter (n = 6). Magnetic bead-labeled white blood cells (green) are deflected into the central core of the sorting channels, away from CTCs (red), thereby allowing continuous depletion of WBCs without clogging the channel. Source data are provided as a Source Data file.” Reproduced from Mishra, A., Huang, SB., Dubash, T. et al. Tumor cell-based liquid biopsy using high-throughput microfluidic enrichment of entire leukapheresis product. Nat Commun 16, 32 (2025). under a CC BY 4.0 Attribution 4.0 International license
- Leukapheresis: A procedure collecting approximately 6 liters of blood, from which leukopaks are derived.
- Debulking: Using size-based inertial flow dynamics, this step removes 99.95% of RBCs and 99.98% of platelets, isolating nucleated cells (WBCs and CTCs).
- Magnetic Sorting: Cells are tagged with biotinylated antibodies against common leukocyte markers and subjected to ultra-high gradient magnetic sorting, achieving remarkable depletion of WBCs and highly purified CTCs.
In a study involving seven patients with metastatic cancers, this platform achieved unparalleled CTC yields (average of 10,057 cells per patient), with a notable 99.96% WBC depletion. Noteworthy findings included:
- Cellular Diversity: Multispectral imaging revealed significant variability in CTC size and marker expression within patients, highlighting the heterogeneity of cancer.
- Molecular Insights: Paired single-cell DNA and RNA sequencing provided a detailed profile of CTCs, revealing tumor-specific genetic alterations, such as aneuploidy, and transcriptional signatures associated with drug resistance.
This innovative platform transforms the landscape of liquid biopsies by enabling high-yield, epitope-agnostic enrichment of intact CTCs. It opens avenues for comprehensive molecular analysis at an unprecedented scale, facilitating personalized cancer management strategies and advancing research into tumor heterogeneity and resistance mechanisms.
“Leukapheresis-based CTC analysis may enable many clinical applications that have previously been limited by the small numbers of CTCs present in 10-20 mL of blood and allow a comprehensive approach to liquid biopsies that will help pave the way for more effective and personalized cancer management strategies.. “, the authors concluded
Figures are reproduced from Mishra, A., Huang, SB., Dubash, T. et al. Tumor cell-based liquid biopsy using high-throughput microfluidic enrichment of entire leukapheresis product. Nat Commun 16, 32 (2025). https://doi.org/10.1038/s41467-024-55140-x under a CC BY 4.0 Attribution 4.0 International license.
Read the original article: Tumor cell-based liquid biopsy using high-throughput microfluidic enrichment of entire leukapheresis product
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


