20 Apr Advancing Cardiac Tissue Engineering: Microfluidic Systems and Heart ECM Integration
Recent advancements in the field of tissue engineering have yielded a novel culture system that significantly enhances the fabrication of human cardiac tissues. This system integrates a microfluidic platform with a heart extracellular matrix (HEM) hydrogel, designed to foster the maturation of tissues that more closely mimic the human cardiac microenvironment.
“The applications of cardiac tissues derived from human pluripotent stem cells are often limited due to their immaturity and lack of functionality. Therefore, in this study, we establish a perfusable culture system based on in vivo-like heart microenvironments to improve human cardiac tissue fabrication. The integrated culture platform of a microfluidic chip and a three-dimensional heart extracellular matrix enhances human cardiac tissue development and their structural and functional maturation.“, the authors explained.
The study details a culture system that supports the growth of cardiovascular lineage cells: cardiomyocytes (CMs), cardiac fibroblasts (CFs), and vascular endothelial cells (ECs), all derived from human induced pluripotent stem cells. The three-dimensional HEM hydrogel, developed from decellularized porcine heart tissue, maintains key extracellular matrix components that are crucial for cellular differentiation and tissue maturation.
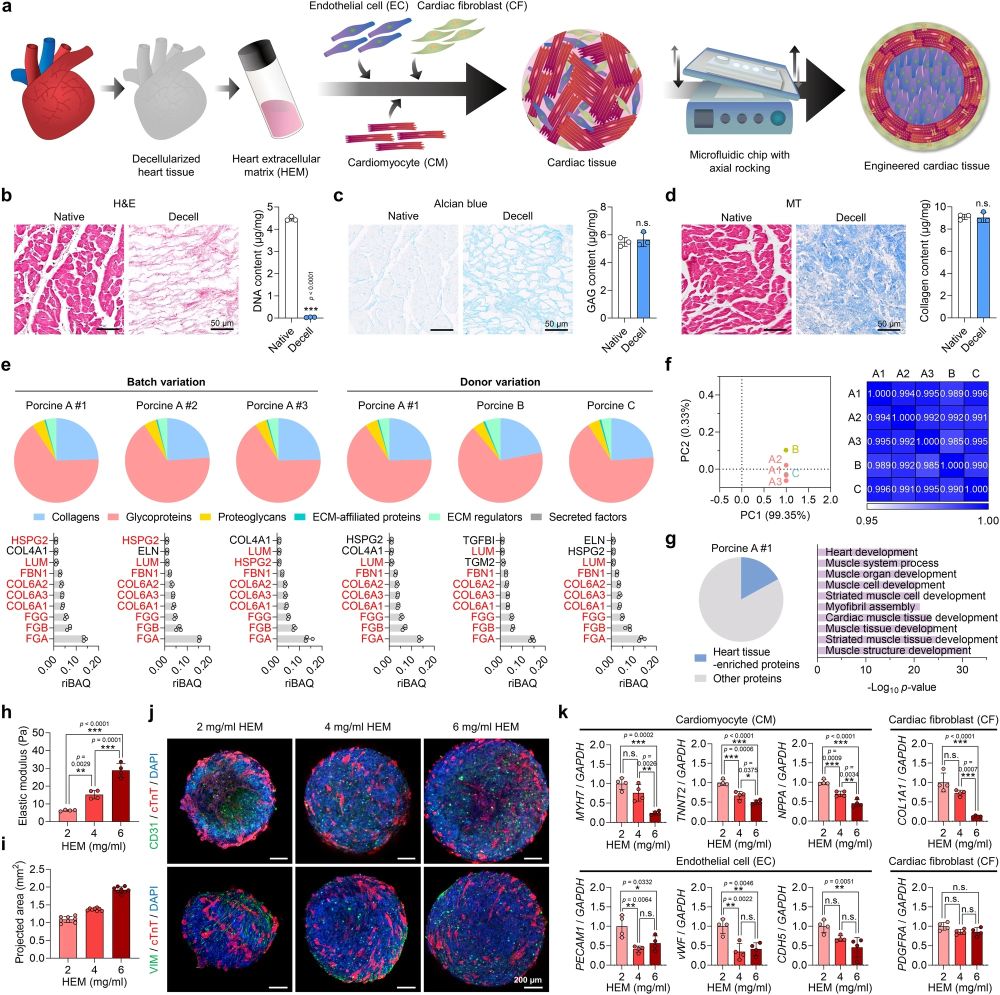
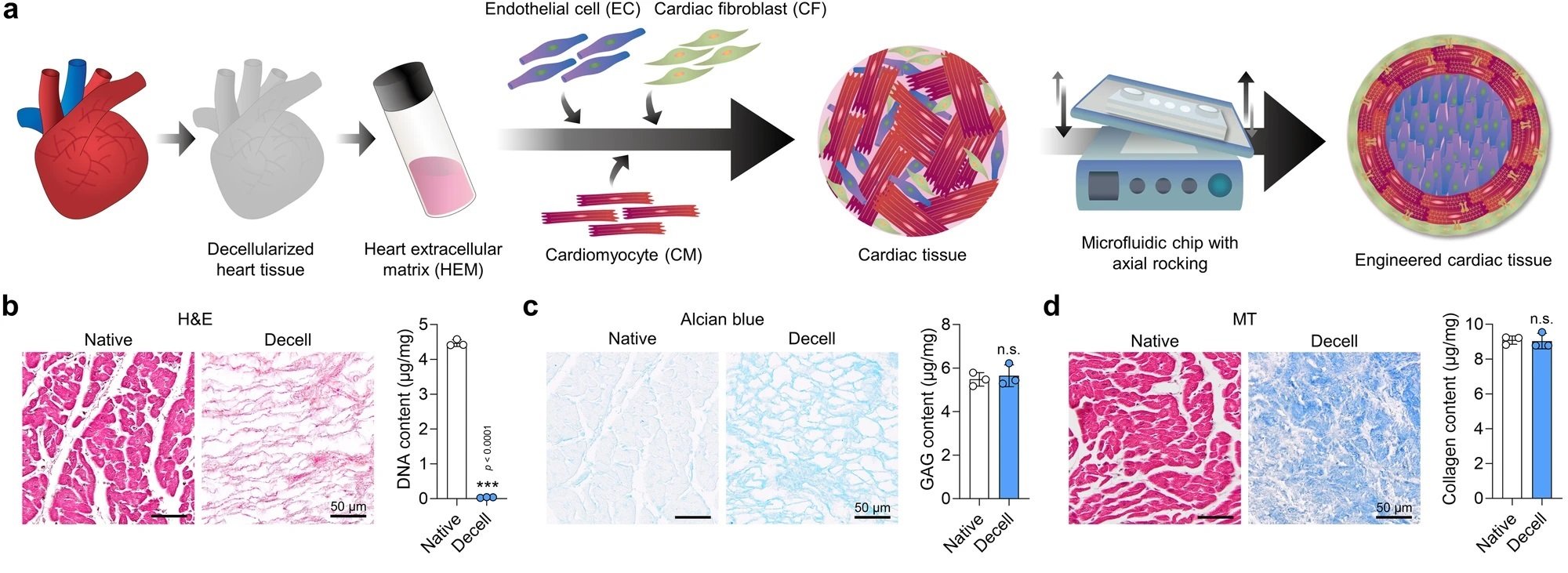
“a Schematic illustration of human cardiac tissue fabrication using three cell types, heart extracellular matrix (HEM) hydrogel, and a microfluidic chip. b Hematoxylin and eosin (H&E) staining and DNA content, c Alcian blue staining and glycosaminoglycan (GAG) content, and d Masson’s trichrome (MT) staining and collagen content of native and decellularized porcine heart tissues (scale bars = 50 μm, N = 3, biological replicates, ***p < 0.001). e–g Proteomic analysis of decellularized porcine tissue-derived extracellular matrix (ECM) samples. e ECM composition and the 10 most abundant matrisome proteins in porcine HEM hydrogel samples derived from different batches (A#1, A#2, and A#3) and different donors (A, B, and C) (N = 3, biological replicates). Red letters represent proteins that overlapped between different batches or donors. f Principal component analysis and Pearson’s correlation analysis of total proteins contained in different HEM samples (A#1, A#2, A#3, B, and C). The mean values of three biological replicates were used for analysis. g Percentage of heart tissue-enriched proteins (4-fold higher than in other tissues) among total proteins contained in the porcine HEM A#1 sample (left) and Gene Ontology Biological Processes (GOBP) analysis of the enriched proteins (right). Fisher’s exact test with false discovery rate correction was used for data analysis. h Elastic moduli of HEM hydrogels produced at concentrations of 2, 4, and 6 mg/ml (N = 4, biological replicates, **p < 0.01 and ***p < 0.001). i The projected areas of cardiac tissues fabricated using 2, 4, and 6 mg/ml HEM hydrogel (N = 8, biological replicates). j Immunofluorescent images of cardiomyocyte (CM; cTnT), endothelial cell (EC; CD31), and cardiac fibroblast (CF; VIM) markers in each group of cardiac tissues (scale bars = 200 μm). k Relative mRNA expression levels of CM (MYH7, TNNT2, and NPPA), EC (PECAM1, vWF, and CDH5), and CF (COL1A1 and PDGFRA) markers in each group of cardiac tissues (N = 4, biological replicates, *p < 0.05, **p < 0.01, ***p < 0.001, data are representative of two independent experiments). Cardiac tissues prepared using human induced pluripotent stem cell (hiPSC)-derived CMs, CFs, and human umbilical vein endothelial cells (HUVECs) in (i–k) were cultured for 7 days and used for analysis. Data are presented as means ± S.D. Statistical significance was determined using unpaired two-sided Student’s t-tests in (b–d) and one-way ANOVA with Tukey’s multiple comparisons tests in (h and k). Non-significant statistical differences are indicated as n.s. (p > 0.05).” Reproduced from Min, S., Kim, S., Sim, WS. et al. Versatile human cardiac tissues engineered with perfusable heart extracellular microenvironment for biomedical applications. Nat Commun 15, 2564 (2024). under a CC BY 4.0 DEED Attribution 4.0 International license.
The engineered cardiac tissues demonstrate substantial improvements in both structure and function. The integration of cell types in a dynamic flow system contributes to an environment that significantly advances the maturation of the cardiac tissues over traditional static culture methods. For instance, macroscale tissues (1-1.2 mm in thickness) maintained in the microfluidic device exhibit enhanced structural and functional properties due to improved nutrient and oxygen delivery facilitated by the dynamic flow.
The functionality of these tissues was validated through extensive testing, including:
Immunofluorescence Staining: Used to assess the presence and distribution of specific cellular proteins, confirming the cellular composition and maturation state of the tissues.
Proteomic Analysis: Provided insights into the protein expressions within the tissues, comparing them with native heart tissues to confirm the physiological relevance of the engineered tissues.
Gene Expression Profiling: Evaluated the expression levels of genes associated with cardiac maturation and functionality, further substantiating the enhanced maturity of the engineered tissues.
The HEM hydrogel demonstrated excellent biocompatibility, with endotoxin levels significantly below FDA thresholds (0.269 ± 0.003 EU/ml). These tests confirm the efficacy of the microfluidic chip and HEM hydrogel system in producing cardiac tissues that are not only structurally and functionally mature but also robust enough for practical applications in clinical research and therapy. The system’s utility spans several biomedical applications:
Drug Testing: The cardiac tissues are used to evaluate cardiotoxicity, showing differential responses to small molecules with varied levels of arrhythmia risk.
Disease Modeling: Conditions like Long QT Syndrome and cardiac fibrosis are effectively modeled, providing new insights into disease mechanisms and potential treatments.
Regenerative Therapy: The tissues show promising results in myocardial infarction treatment models, suggesting potential for regenerative medicine applications.
This study represents a significant step forward in the field of tissue engineering, providing a robust platform that not only enhances the maturation of human cardiac tissues but also offers a scalable solution for their application in drug testing, disease modeling, and regenerative therapies. The integration of a microfluidic chip and HEM hydrogel creates a more physiologically relevant model that could have substantial impacts on future research and therapeutic approaches.
“We anticipate that our versatile cardiac tissues will be established as practically available human in vitro cardiac models and provide therapeutic regimens for in vivo cardiac regeneration through further translational research“, the authors concluded.
Figures are reproduced from Min, S., Kim, S., Sim, WS. et al. Versatile human cardiac tissues engineered with perfusable heart extracellular microenvironment for biomedical applications. Nat Commun 15, 2564 (2024). https://doi.org/10.1038/s41467-024-46928-y under a CC BY 4.0 DEED Attribution 4.0 International license.
Read the original article: Versatile human cardiac tissues engineered with perfusable heart extracellular microenvironment for biomedical applications
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


