18 Sep Advancements in Chronic Wasting Disease Detection with Microfluidic Devices
Chronic Wasting Disease (CWD) stands as a formidable challenge in wildlife conservation, a silent predator threatening populations across the globe. Yet, amid this crisis emerges a beacon of hope – a Microelectromechanical Systems (MEMS) biosensor, intricately intertwined with the realms of microfluidic fabrication and precision engineering. In this article, we delve into an innovative study that has paved the way for a more efficient and sensitive detection method, promising hope for the future of CWD control. Join us on this journey as we delve deep into the revolutionary potential of microfluidic technology in the realm of CWD detection and wildlife disease management.
“We have investigated an accurate, rapid, and low-cost microfluidic microelectromechanical system (MEMS) biosensing device for the detection of CWD pathologic prions in retropharyngeal lymph nodes (RLNs), which is the current standard type of CWD diagnostic sample.“, the authors explained.
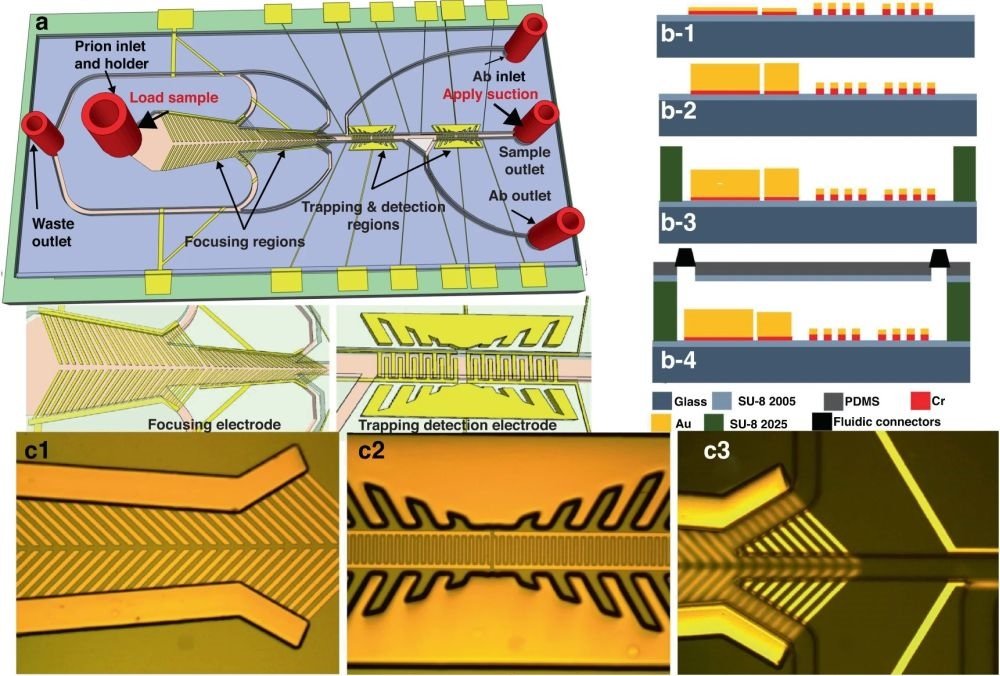
At the heart of this MEMS biosensor lies the marvel of microfluidic chips. The integration of microfluidic channels enables the precise control of fluid flow and the manipulation of minuscule volumes, paving the way for enhanced sensitivity and accuracy in CWD prion detection. Behind every great innovation, there’s meticulous craftsmanship. The microfabrication process is where the biosensor takes shape, with exacting steps such as cleaning, photoresist coating, and electrode deposition ensuring optimal functionality. This level of precision sets the stage for the biosensor’s remarkable performance. To assess the microfluidic device’s ability to concentrate CWD prion proteins, researchers employed fluorescent dielectric latex nanobeads. These nanobeads, with diameters ranging from 200 nm to < 1 µm, were used as surrogates for prion proteins. Using a fluorescence microscope equipped with digital light-sheet and diode lasers, researchers meticulously observed the movement of these nanobeads within the microchannels. An optimum AC signal of 4 Vp-p at 5 MHz was applied to the focusing electrode, compelling the nanobeads to migrate towards the microchannel’s centerline. The results were nothing short of impressive. Fluorescent images captured before and after focusing revealed the nanobeads perfectly aligned at the microchannel’s center, concentrated and immobilized atop the detection electrode. This precision bodes well for the detection of CWD prions.
“The workflow of smRandom-seq for microbe samples includes fixation, cell wall digestion, reverse transcription, dA tailing, droplet barcoding, primers releasing and extension, droplets breaking and PCR amplification, and Cas9-based rRNA depletion and sequencing. Blue dashed box: the two in situ reactions, including reverse transcription and dA tailing. AAA: dA tail in the 3’ of cDNA, TTT: poly(dT) in the barcoded primers.” Reproduced from Xu, Z., Wang, Y., Sheng, K. et al. Droplet-based high-throughput single microbe RNA sequencing by smRandom-seq. Nat Commun 14, 5130 (2023). under Creative Commons Attribution 4.0 International License.
The next crucial step involved optimizing the concentration of monoclonal antibodies (mAb) for enhanced sensitivity. Multiple antibody dilutions, ranging from 0.25 µg/mL to 10 µg/mL, were prepared. The microchannel was meticulously cleaned, and antibody impedance was recorded after each binding event. Results were intriguing – the highest impedance signal was not obtained at the highest antibody concentration but rather at 2 µg/mL. This finding reduced the overall cost of the sensor while maintaining performance. Further optimization focused on antibody coating time. Researchers found that an incubation time of 1 to 1.5 hours yielded optimal results. Beyond this timeframe, impedance values did not significantly improve, making this the ideal coating duration.
Now, let’s talk sensitivity. The PDMS-based microfluidic device demonstrated its prowess by detecting engineered prion antigens at a dilution of 1:24, outperforming the widely used ELISA method, which only detected the same antigen at a dilution of 1:8. The biosensor also exhibited a relative limit of detection (rLOD) of 1:1000 dilution for strong positive retropharyngeal lymph node (RLN) samples, surpassing ELISA’s rLOD of 1:100. These results were a resounding success, indicating that the microfluidic device is not just another tool in the arsenal but a significant leap forward in sensitivity and disease detection.
The microfluidic device’s specificity and selectivity are nothing short of remarkable. When tested against unrelated pathogens like Bluetongue (BT) and Epizootic hemorrhagic disease (EHD) viruses, the biosensor held its ground, registering no impedance changes compared to the baseline antibody values. This showcased its exceptional specificity. Additionally, selectivity was assessed by pitting anti-prion mAb against anti-Bovine Coronavirus (BCV) mAb. The results spoke volumes – the biosensor’s impedance response to prion antigen was significantly higher, while signals from the antigen/anti-BCV antibody interaction barely budged. It’s clear that the microfluidic device is highly selective in distinguishing between prion and unrelated antibodies.
“a A cartoon showing the experimental setup. Top-view cartoon showing the flow direction during: b-1 antibody coating where it was first placed at the antibody inlet and suction was applied to the antibody outlet while all other inlets were closed, b-2 CWD prion antigen loading at the sample inlet while suction was applied to the waste outlet. The flow continued toward the detection region. The process flow for antibody immobilization, and the antibody/ antigen binding on the interdigitated microelectrode: c-1 the antibody was loaded from the antibody inlet while suction was applied from the antibody outlet, c-2 the microchannel was washed after adhesion of antibody to the IDE array, c-3 the CWD prion protein sample was loaded into the sample inlet while suction was applied to the sample outlet, c-4 the microchannel was washed again after antibody antigen binding was completed, d a package biosensor” Reproduced from Muhsin, S.A., Abdullah, A., kobashigawa, E. et al. A microfluidic biosensor for the diagnosis of chronic wasting disease. Microsyst Nanoeng 9, 104 (2023). under Creative Commons Attribution 4.0 International License.
To leave no room for doubt, researchers conducted tests to confirm that impedance changes were indeed a result of pathogenic prion detection. Pathogenic prions are notoriously resistant to proteases, setting the stage for a compelling experiment. RLN samples were treated with proteinase K to eliminate nonpathogenic prion proteins. The results were unequivocal – impedance values for both untreated and proteinase K-treated RLN samples were virtually identical. This confirmed that the impedance changes were solely due to the detection of pathogenic prions, ruling out any influence from matrix effects or nonpathogenic prion proteins.
As promising as these results are, the journey is far from over. The researchers envision the development of a portable biosensing system that can make this technology accessible to a broader audience. This system will encompass all the essential components, from electrical circuits to data analysis software, making it user-friendly and efficient.
“To translate the device into practice in the form of a commercial device, a small portable biosensing system will be built, which can be used to read the signal. The system will include the electrical circuits (e.g., impedance circuit to replace the impedance analyzer), the data analysis software, the hardware, pump, sample and waste holders, and professional-grade data display where the user can enter sample information using a local keypad or keyboard to achieve the full feature (e.g., integration of the biosensor system to smartphone), commercially viable prototype. “, the authors explained.
In conclusion, this research exemplifies the potential of microfluidic devices and microfabrication techniques in disease detection. With remarkable sensitivity, specificity, and selectivity, the microfluidic device offers hope in the fight against CWD, ultimately enhancing wildlife management and disease control. In the relentless battle against CWD, microfluidics stands as a beacon of hope, offering a brighter future for deer populations and wildlife enthusiasts alike. Stay tuned for more breakthroughs in this pioneering field!
Remember to check our blog for more exciting research highlights and stay up to date with the latest advancements in science and technology!
Figures are reproduced Muhsin, S.A., Abdullah, A., kobashigawa, E. et al. A microfluidic biosensor for the diagnosis of chronic wasting disease. Microsyst Nanoeng 9, 104 (2023). https://doi.org/10.1038/s41378-023-00569-1 under a Creative Commons Attribution 4.0 International License)
Read the original article: A microfluidic biosensor for the diagnosis of chronic wasting disease


