26 Nov A microfluidic platform facilitates high-throughput mechanical testing of single molecules
Abstract
“Current approaches for single molecule force spectroscopy are typically constrained by low throughput and high instrumentation cost. Herein, a low-cost, high throughput technique is demonstrated using microfluidics for multiplexed mechanical manipulation of up to ~4000 individual molecules via molecular fluid loading on-a-chip (FLO-Chip). The FLO-Chip consists of serially connected microchannels with varying width, allowing for simultaneous testing at multiple loading rates. Molecular force measurements are demonstrated by dissociating Biotin-Streptavidin and Digoxigenin-AntiDigoxigenin interactions along with unzipping of double stranded DNA of varying sequence under different dynamic loading rates and solution conditions. Rupture force results under varying loading rates and solution conditions are in good agreement with prior studies, verifying a versatile approach for single molecule biophysics and molecular mechanobiology. FLO-Chip enables straightforward, rapid, low-cost, and portable mechanical testing of single molecules that can be implemented on a wide range of microscopes to broaden access and may enable new applications of molecular force spectroscopy.”

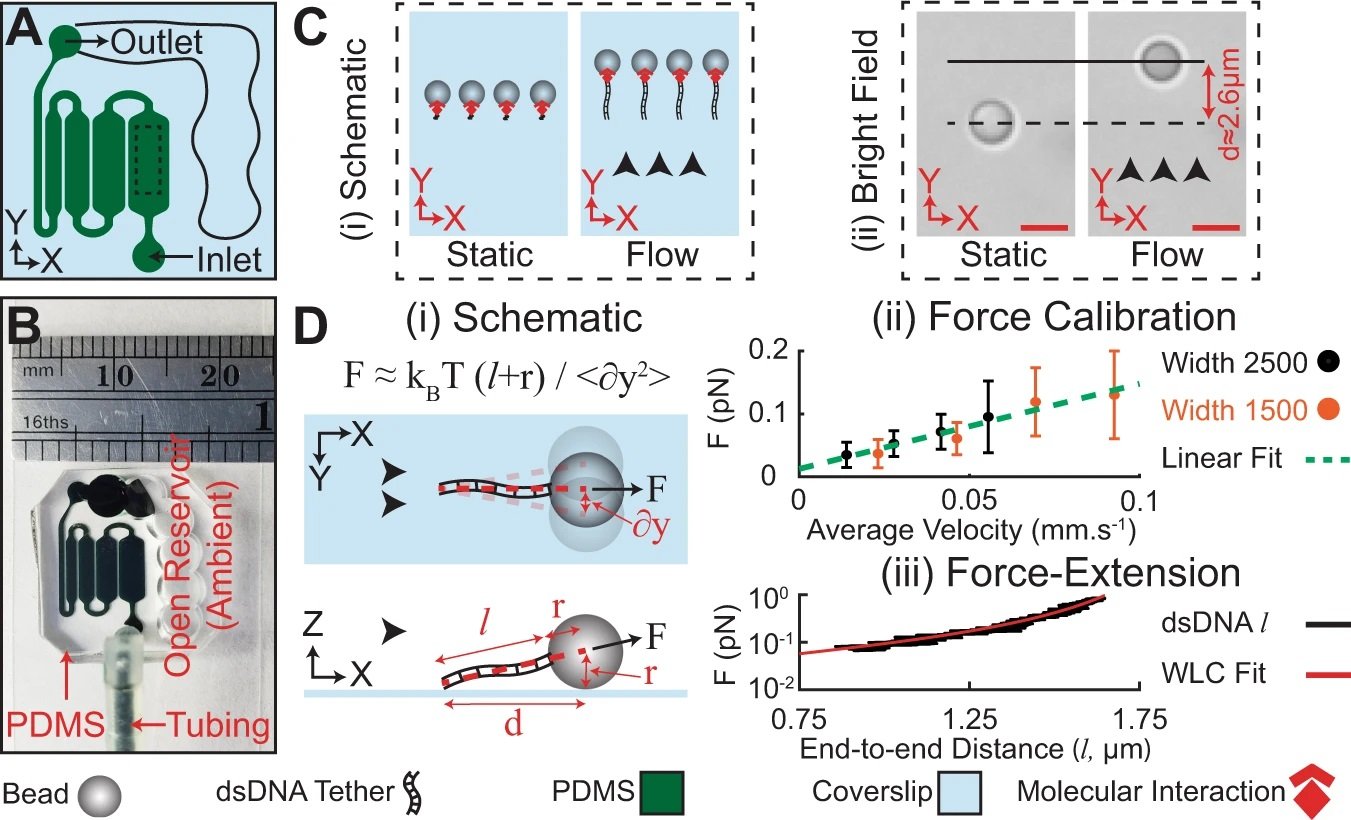
“A, B Schematic and representative photograph of the microfluidic platform used in fluid loading on a chip (FLO-Chip). C (i) Schematic, and (ii) Differential Interference Contrast (DIC) image of beads anchored to the coverslip via a single dsDNA tether. Application of flow causes ~2.6μm displacement of the bead center as the result of the drag force applied on the bead. Scale bars, 2 μm. D (i) Schematic of the direction of in-plane and off-plane stretching force (F) applied on a tether bead under flow. Application of F leads to transverse fluctuation of the tethered bead. (ii) Calibration of F with respect to the inlet perfusion rate (Q) in the 1500 μm and 2500 μm wide microchannel. Transverse fluctuation of n = 33 beads in the 2500 µm wide channel and n = 23 beads in the 1500 µm wide channel were monitored over three independent experiments under different flow rates. Error bars represent standard deviation. The calibration line (red dash line) indicated a linear relationship between drag force and perfusion rate with slope of ~1.4 ± 0.2 pN s mm−1. Error bars represent standard deviation. (iii) Force-extension examination of single tethers under varying F. The tether length (l) was well described according to the worm-like chain model (solid red line, persistence length = 56 ± 8 nm, contour length = 1.8 ± 0.1 µm). Black arrowheads denote flow direction. Scale bars are 2 μm. Source data are provided as a Source Data file.” Reproduced under a Creative Commons Attribution 4.0 International License from Akbari, E., Shahhosseini, M., Robbins, A. et al. Low cost and massively parallel force spectroscopy with fluid loading on a chip. Nat Commun 13, 6800 (2022).
Figures and the abstract are reproduced from Akbari, E., Shahhosseini, M., Robbins, A. et al. Low cost and massively parallel force spectroscopy with fluid loading on a chip. Nat Commun 13, 6800 (2022). https://doi.org/10.1038/s41467-022-34212-w under a Creative Commons Attribution 4.0 International License.
Read the original article: Low cost and massively parallel force spectroscopy with fluid loading on a chip


