04 Mar A microfluidic approach for studying integrin αIIbβ3 activation and clustering
“Platelet adhesion and activation are mediated by integrin αIIbβ3 clustering, which is crucial for the hemostatic function of platelets. In an activated state, integrins provide the connection between the extracellular matrix and the actin cytoskeleton through a variety of cytoplasmic proteins, such as talin. Here, droplet-based microfluidics is applied to generate cell-sized giant unilamellar vesicles (GUVs) with a defined molecular composition to quantify the adhesion of integrin αIIbβ3-containing protocells in relation to the number of integrin–talin head domain (THD) complexes. Furthermore, it is shown that THD induces integrin clustering in protocells adhering to fibrinogen. The formation of this molecular link, which has, so far, only been observed in vivo, is an essential step in synthetic cell design to recapitulate integrin-mediated bidirectional signaling across the membrane. These results pave the way for further quantitative investigations of protein–protein interactions between integrins and associated proteins and their assembly within such defined, but complex, synthetic cells. An essential future step to mimic the complex interaction between cells and their environment will be to combine synthetic approaches with peptide chemistry to guide the molecular mechanisms involved in integrin binding and activation.”

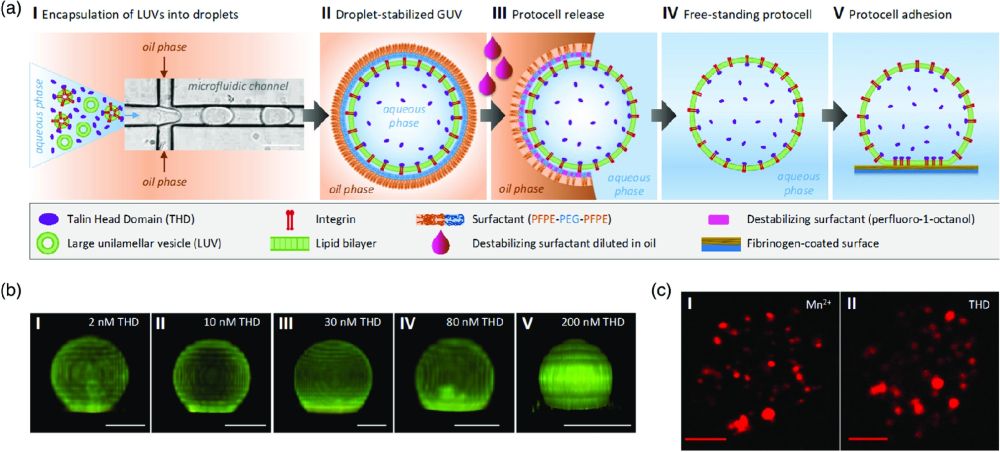
“Formation and adhesion of protocells containing integrin and THD. a) Schematic depiction of the formation of functional cell-sized liposomes that contain integrin and THD by droplet-based microfluidics. Scale bar: 50 μm. I) Encapsulation of bare liposomes (≈100 nm diameter), integrin-reconstituted proteoliposomes, and THD at defined concentration into microfluidic water-in-oil droplets of 30 μm diameter. II) Liposomes rupture at the droplets’ inner interface, fuse, and form a continuous lipid bilayer, the dsGUV. III) After the addition of destabilizing surfactant, the GUV is released from the stabilizing oil phase into an aqueous phase, i.e., an appropriate buffer solution. IV) Free-standing GUV that contains integrin and THD of defined concentrations, after its release. V) Released GUV adhering to a fibrinogen-coated surface as a result of integrin-activation by THD. Depiction not to scale. b) 3D-reconstitutions of integrin-containing protocells (lipid: ATTO488 (green), integrin: Alexa568 (red)). The images show increasing adhesion to fibrinogen-coated surfaces in correlation to an initially provided THD concentration of: I) 2 nM, II) 10 nM, III) 30 nM, IV) 80 nM, V) 200 nM at a constant integrin concentration. The spreading value of each vesicle shown is close to the mean value obtained from a corresponding sample of vesicles. For correlating data refer to Table 1. Scale bar: 10 μm. c) Integrin-clustering in the contact area of protocells adhering to fibrinogen-coated surfaces. The lipid composition of the protocells is 18:72:10 DOTAP:DOPC:Egg PC with reconstituted integrin (Alexa568) activated by: I) 1 mM Mn2+/1 mM Mg2+ (vesicle diameter: 12 μm) and II) 200 nM THD (vesicle diameter: 14 μm). The difference in the overall signal and concentration of clusters can be due to bleaching effects. Scale bar: 3 μm”. Reproduced under Creative Commons Attribution 4.0 International License from Benk, L.T., Benk, A.S., Lira, R.B., Cavalcanti-Adam, E.A., Dimova, R., Lipowsky, R., Geiger, B. and Spatz, J.P. (2022), Integrin αIIbβ3 Activation and Clustering in Minimal Synthetic Cells. Adv. NanoBiomed Res. 2100094.
Figures and the abstract are reproduced from Benk, L.T., Benk, A.S., Lira, R.B., Cavalcanti-Adam, E.A., Dimova, R., Lipowsky, R., Geiger, B. and Spatz, J.P. (2022), Integrin αIIbβ3 Activation and Clustering in Minimal Synthetic Cells. Adv. NanoBiomed Res. 2100094. https://doi.org/10.1002/anbr.202100094 under Creative Commons Attribution 4.0 International License
Read the original article: Integrin αIIbβ3 Activation and Clustering in Minimal Synthetic Cells


