29 Jul Advancing Nanoparticle Design: Microfluidic Synthesis of Complex Liposomes
Recent advancements in microfabrication of microfluidic chips are pushing the boundaries of nanoparticle design, offering new possibilities for drug delivery and biotechnology applications. A team of researchers has developed a novel method for creating nanoscale liposomes with enhanced structural complexity, addressing longstanding limitations in the field.
Existing soft-matter nanoparticles, such as liposomes, have been constrained by their simplistic structures, typically consisting of a single membrane-bound aqueous compartment. This lack of architectural complexity has limited their functional scope, particularly in areas requiring precise control over multiple payload release or compartmentalized reactions.
“Given that form and function are intertwined, this lack of architectural complexity restricts the development of more sophisticated properties. To address this, we have devised an engineering strategy combining microfluidics and conjugation chemistry to synthesize nanosized liposomes with two discrete compartments, one within another, which we term concentrisomes “, the authors explained.

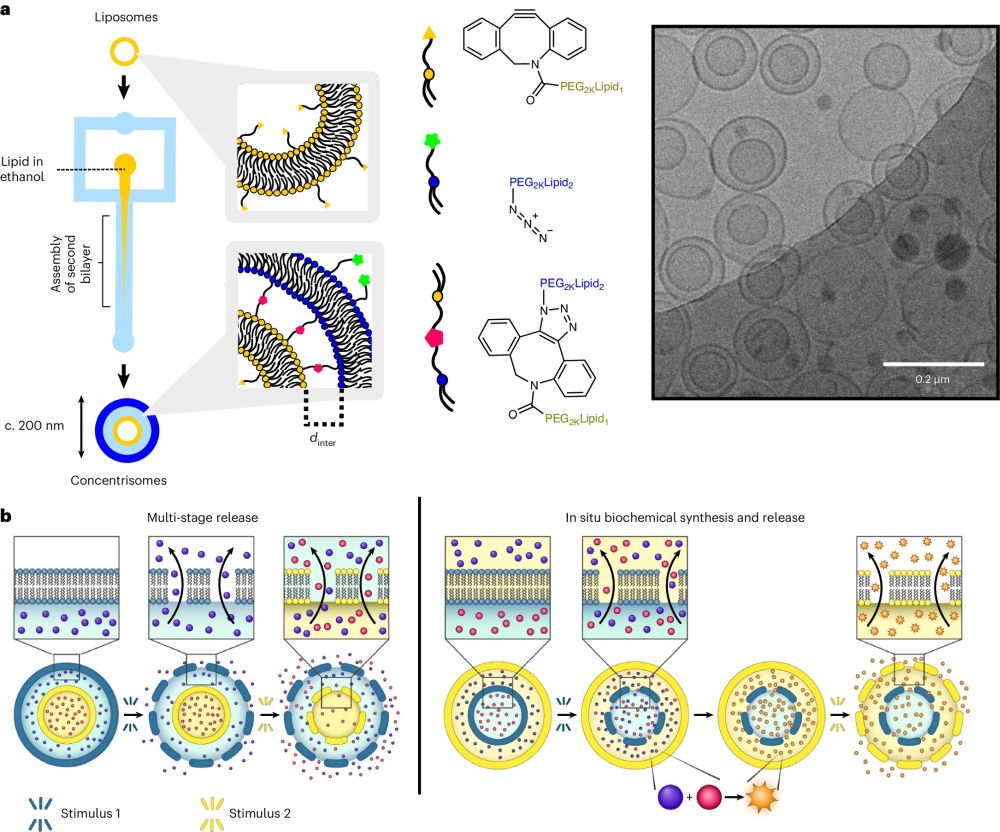
“a, A graphic illustrating the steps involved in the click-chemistry-assisted microfluidic synthesis of concentrisomes. A liposome suspension with alkyne moieties is fed into a MHF chip as a sheathing buffer stream, which is co-flowed alongside a secondary lipid composition that contains azide-functionalized lipids dissolved in ethanol. Micellar/bicellar aggregates tether to the external leaflet of pre-formed vesicles and eventually close to generate a second bilayer. The DBCO moiety, azide and triazole product are represented by a yellow triangle, green star and pink pentagon, respectively. The corresponding chemical structures are shown to the right of each graphic. A representative cryo-TEM micrograph of the concentrisome (double-bilayer liposome) is given on the right. Scale bar, 200 nm. b, A graphical illustration of possible concentrisome functionality: multi-cargo encapsulation in different compartments, triggered sequential multi-stage release of two different payloads, and in situ biochemical synthesis within the attolitre volume of the particle itself, followed by release.” Reproduced from Pilkington, C.P., Gispert, I., Chui, S.Y. et al. Engineering a nanoscale liposome-in-liposome for in situ biochemical synthesis and multi-stage release. Nat. Chem. (2024). under a CC BY 4.0 Attribution 4.0 International license
The “concentrisomes” are nanoscale liposomes with two discrete compartments, one nested within another. Their method combines microfluidic hydrodynamic focusing (MHF) with conjugation chemistry, allowing unprecedented control over particle architecture and composition.The concentrisomes exhibited the capability for multi-stage and site-specific release of encapsulated agents. This was achieved by designing bilayers to respond to predetermined external stimuli, thus allowing for the controlled release of substances at specific times and locations. Such a feature is particularly beneficial for applications requiring the simultaneous delivery of multiple drugs with precise timing, such as in combination therapy.
The synthesis process involves two key steps utilizing microfluidic devices:
- Initial liposome formation: Using a microfluidic chip, unilamellar liposomes are created through MHF.
- Outer bilayer addition: A second microfluidic device is employed to add an outer bilayer around the initial liposomes.
The researchers incorporated click chemistry (specifically, strain-promoted azide/alkyne cycloaddition) to facilitate the formation of the outer bilayer and control inter-bilayer spacing. This approach allows precise control over:
- Composition of each bilayer
- Overall particle size
- Dimensions between inner and outer membranes
- Identity of encapsulated cargo within each compartment
- Biophysical features of inner and outer bilayers
The microfluidic synthesis method yielded concentrisomes with several notable properties:
- Multi-stage payload release: By incorporating thermoresponsive lipids into different bilayers, the team demonstrated sequential release of two different payloads at defined time points.
- In situ biochemical synthesis: The researchers created attolitre-scale reactors by encapsulating enzymes and substrates in separate compartments, allowing controlled mixing and reaction upon external stimulation.
- Tunable inter-bilayer spacing: Using different lengths of PEG spacers, the team showed the ability to adjust the distance between the inner and outer bilayers.
- Precise size control: The microfluidic approach consistently produced particles below 200 nm in diameter, a crucial factor for many biomedical applications.
The development of concentrisomes marks a significant step forward in the design of nanoparticle systems for complex drug delivery and synthetic biology applications. Their ability to mimic biological compartmentalization, combined with the precision in functional design, opens up new avenues in targeted therapy and on-demand biochemical processes.
As research in this area progresses, we can expect to see further refinements in microfluidic microfabrication techniques and exploration of diverse applications for these sophisticated nanoparticles. The integration of this technology with other lab-on-chip modules for downstream processes like purification and sterilization presents exciting opportunities for future development in the field of nanomedicine and biotechnology.
Figures are reproduced from Pilkington, C.P., Gispert, I., Chui, S.Y. et al. Engineering a nanoscale liposome-in-liposome for in situ biochemical synthesis and multi-stage release. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01584-z under a CC BY 4.0 Attribution 4.0 International license.
Read the original article: Engineering a nanoscale liposome-in-liposome for in situ biochemical synthesis and multi-stage release
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


