11 Jul Developing Gene Delivery Cargos Using Microfluidic High-throughput Screening
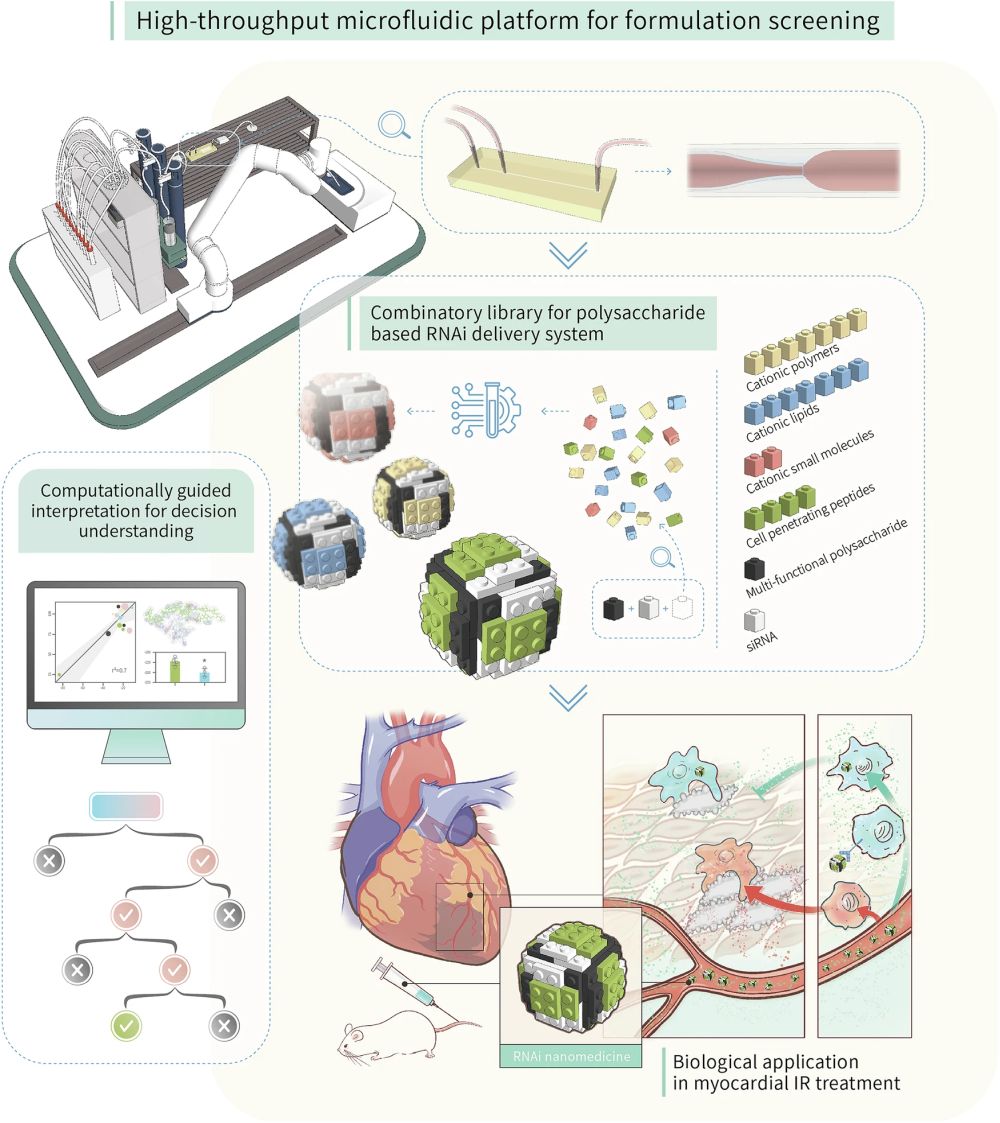
Heart diseases, particularly those caused by ischemia, are a leading cause of morbidity and mortality worldwide. Traditional treatment strategies often fall short in specificity and efficiency, necessitating a more targeted approach. RNAi therapy, which involves silencing specific genes responsible for disease pathology, presents a promising avenue. However, the challenge has always been the effective delivery of RNAi molecules to the heart without affecting other tissues. Researchers have introduced an microfluidic device for developing potent gene delivery cargos by using ionotropic gelation—a process for creating nanoparticles (NPs) with polysaccharides. This method is notable for its simplicity and environmental friendliness, but it requires optimization to improve its efficacy specifically for heart disease. The new study utilizes a robot-assisted microfluidic chip for diversity-oriented combinatorial formulation screening, enabling the identification of the most effective combinations from a vast library of cationic compounds and polysaccharides.
“Herein, we report a diversity-oriented combinatory formulation screen scheme to identify potent gene delivery cargo in the context of precision cardiac therapy. Distinct categories of cationic compounds are tested to construct RNA delivery system with an ionic polysaccharide framework, utilizing a high-throughput microfluidics workstation coupled with streamlined NPs characterization system in an automatic, step-wise manner. “, the authors explained.

“The current research arose with the construction of cationic compounds collections with distinct categories, and a previously synthesized multi-functional polysaccharide was fixed as ionic part. The screen process was aided by a high-throughput microfluidics workstation coupled with streamlined NPs characterization system, and the step-wise screen was interpreted by a computational aided analysis for potentially understanding the decision-making mechanism. In the end, the out-of-bag RNAi nanomedicine was tested in a murine myocardial reperfusion model to evaluate its corresponding therapeutic potency.” Reproduced from Gao, H., Li, S., Lan, Z. et al. Comparative optimization of polysaccharide-based nanoformulations for cardiac RNAi therapy. Nat Commun 15, 5398 (2024). under a CC BY 4.0 Attribution 4.0 International license
The core of the experimental innovation lies in the use of a high-throughput microfluidics workstation, integrated with a streamlined nanoparticle characterization system. This setup allowed for the rapid screening and optimization of numerous nanoformulations. One of the standout formulations identified through this method was named GluCARDIA. It demonstrated superior cardiac accumulation and gene silencing efficacy in a murine model of myocardial ischemia/reperfusion injury.
The high-throughput microfluidic device employed in this study facilitated the rapid screening and optimization of diverse nanoformulations. This microfluidic platform consists of several key components:
1- Sample Preparation Module: This part of the device handles the initial preparation of the samples, allowing for precise dosing and mixing of the polysaccharide (EEPG) with various cationic compounds. The samples are prepared in a buffer solution and loaded into the system.
2- Microfluidic Mixing Module: The core of the microfluidic chip , this module consists of a series of microchannels and microfluidic chambers where the actual mixing of the polysaccharide with cationic materials takes place. The design of these microfluidic channels ensures efficient and controlled mixing, critical for the formation of nanoparticles through ionotropic gelation.
3- Analysis Module: Integrated with dynamic light scattering (DLS) instruments, this part of the device allows for the immediate analysis of the formed nanoparticles. Parameters such as particle size, zeta potential, and encapsulation efficiency are measured in real-time, providing immediate feedback for optimization.
Operationally, the device automates the entire process from sample preparation to analysis. It is equipped with pumps and valves that are computer-controlled to manage fluid flow at precise rates. This precision allows for the consistent production of nanoparticles under standardized conditions, which is vital for ensuring the reproducibility of the nanoformulations.
GluCARDIA nanoparticles are specifically designed to target Dectin-1, a receptor over-expressed in injured myocardium. This targeting is achieved through the unique properties of the endosomolytic phosphorylated β-glucan (EEPG) used in the formulation, which naturally binds to Dectin-1, allowing for precise delivery of therapeutic agents directly to the heart tissue. GluCARDIA NPs were tested in a clinically relevant murine model of myocardial ischemia/reperfusion injury, showing significantly enhanced cardiac accumulation. This targeting capability was confirmed through extensive in vivo experiments, where GluCARDIA NPs showed a higher heart targeting index compared to conventional nanoparticles, leading to more effective gene silencing and reduced cardiac damage.
Further tests revealed that GluCARDIA NPs not only improved targeting but also maintained high biocompatibility and gene silencing efficacy. They successfully delivered siRNA that suppressed the expression of specific genes involved in inflammatory responses, which are critical in the progression of myocardial injury. The use of these NPs resulted in a marked reduction in inflammatory markers and fibrosis, showcasing their potential to significantly mitigate the effects of cardiac injuries.
The microfluidic device used in this study exemplifies the integration of engineering and biomedical science to push the boundaries of drug delivery technology. Its sophisticated design and operation have enabled the rapid screening and optimization of nanoparticle formulations, leading to the successful development of GluCARDIA NPs. This tool not only enhances the efficiency and precision of research but also paves the way for future developments in nanoformulation technology for various medical applications.
“The concept of the combination of deep-learning neural network-based artificial intelligence and flexible microfluidic colorimetric device presents a promising, convenient, and low-cost strategy for the assessment of ocular and systemic health through non-invasive monitoring of key biomarkers in human tears, providing research directions to advance the field of telehealth and personalized precision medicine.“, the authors concluded.
Figures are reproduced from Gao, H., Li, S., Lan, Z. et al. Comparative optimization of polysaccharide-based nanoformulations for cardiac RNAi therapy. Nat Commun 15, 5398 (2024). https://doi.org/10.1038/s41467-024-49804-x under a CC BY 4.0 Attribution 4.0 International license.
Read the original article: Comparative optimization of polysaccharide-based nanoformulations for cardiac RNAi therapy
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


