21 Feb Integrating Functional Vascularized Organoids-on-Chip
Recent advancements in tissue engineering and organ-on-chip technology have led to the development of a novel microfluidic platform designed for the encapsulation and long-term culture of various types of cell aggregates with an emphasis on vascularization. This microfluidic chip represents a step forward in addressing the challenge of creating functional vascular networks within three-dimensional cell aggregates, a crucial aspect for their survival, functionality, and integration into tissue engineering applications. This post provides a detailed overview of the study’s objectives, methodologies, results, and implications for future research.
“Most existing microfluidic devices poorly reflect the complexity of in vivo flows and require complex technical set-ups. Considering these constraints, we develop a platform to establish and monitor the formation of endothelial networks around mesenchymal and pancreatic islet spheroids, as well as blood vessel organoids generated from pluripotent stem cells, cultured for up to 30 days on-chip.“, the authors explained.
The primary goal of this study was to design and validate a microfluidic platform capable of facilitating the formation and maintenance of endothelial networks around organoids derived from mesenchymal and pancreatic islet spheroids, as well as blood vessel organoids from pluripotent stem cells. Achieving functional vascularization within these organoids is essential for mimicking physiological conditions, thereby enhancing their potential application in drug discovery, disease modeling, and regenerative medicine.
The platform employs hydrodynamic and capillary effects for the encapsulation process, simplifying the vascularization of three-dimensional cell aggregates. This methodological innovation allows for the culture of organoids on-chip for up to 30 days, demonstrating the platform’s utility in supporting long-term experiments. The study outlines the technical considerations involved in optimizing the encapsulation process, including adjustments to the gel layer thickness and strategies to minimize cell loss during encapsulation.
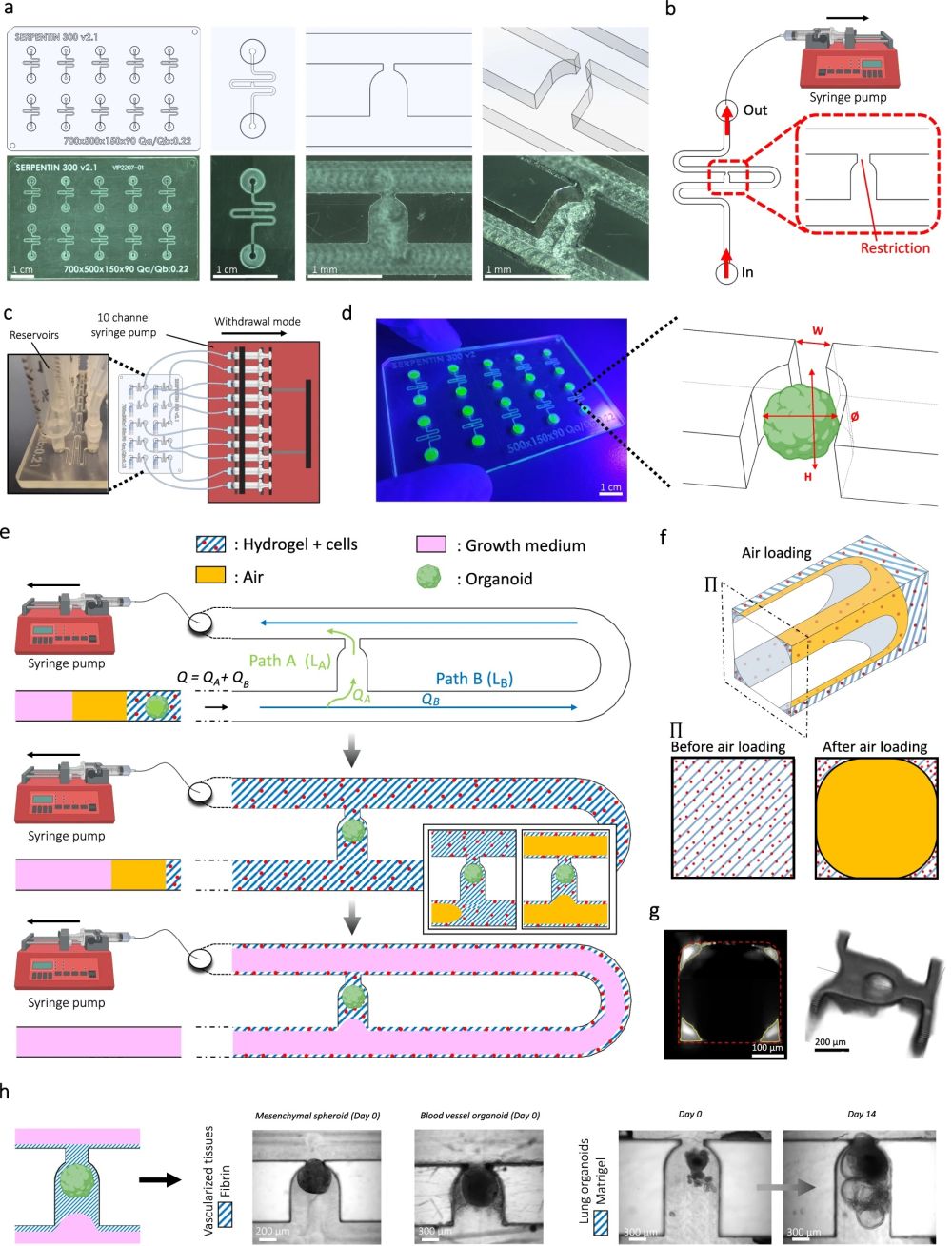
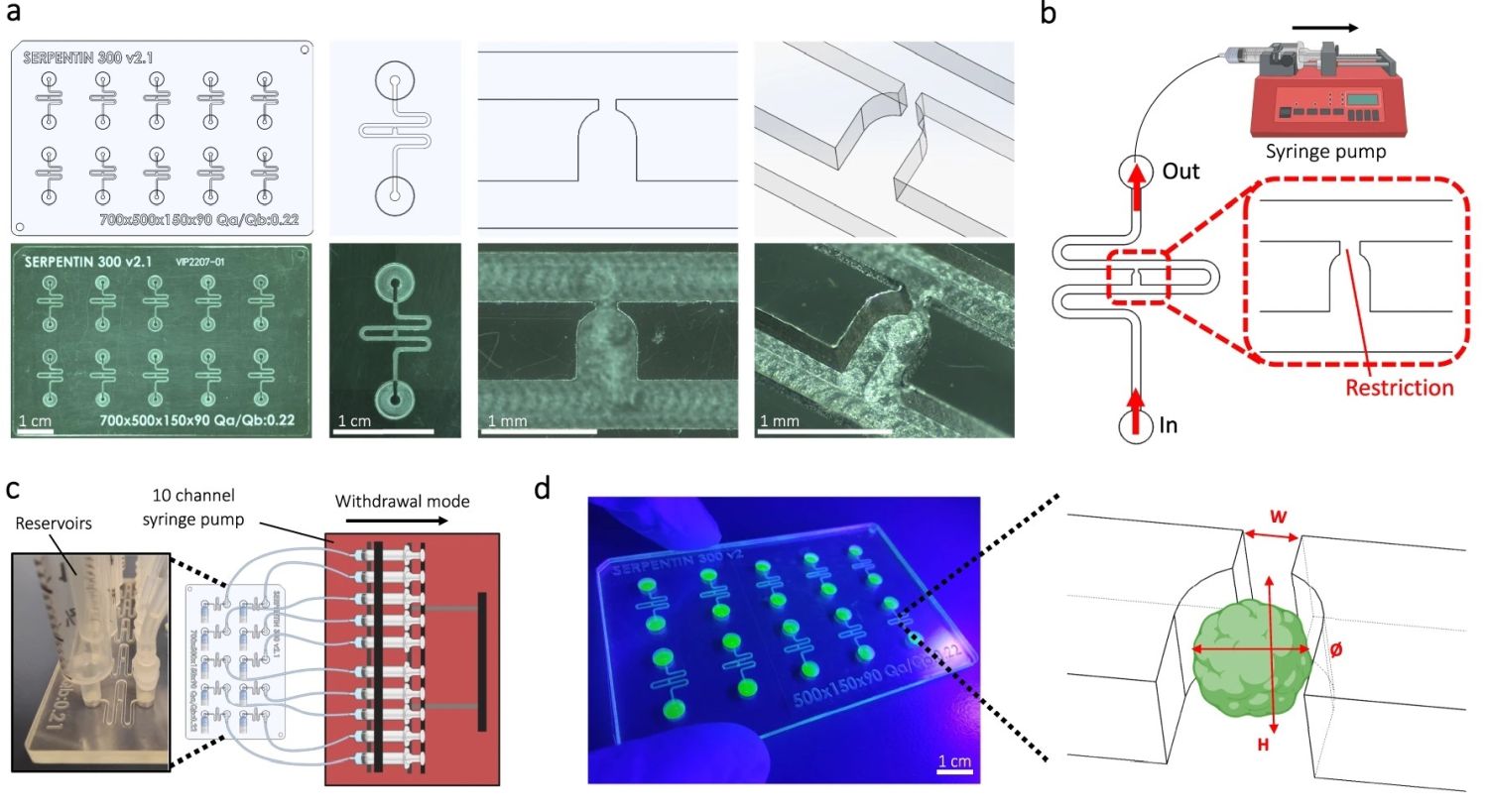
“a Computer-aided design (top) and photographs (bottom) of the microfluidic chip displaying 10 microchannels, with each microchannel featuring a trapping site. b Top view of the microfluidic device. A syringe pump was connected to the outlet of the channel to introduce fluid perfusion. c Schematic diagram and photograph of the parallelization feature of our setup, showcasing 10 microchannels controlled simultaneously. d Photograph of the microfluidic chip and schematic three-dimensional view of the U-cup shaped area functioning as a trap. Here, the trap site is exemplarily occupied by a cell aggregate. e Schematic diagram showing an overview of the loading process. Initially, the hydrogel containing an organoid and HUVEC cells was introduced. Before polymerization of the hydrogel, air was introduced to position the hydrogel and the HUVEC cells. Finally, growth medium was introduced for continuous perfusion of the microfluidic chamber and the trapped organoid. f Schematic 3D and cross-sectional views of the microchannel showing the air loading process and associated hydrogel deposition. g Experimental cross-sectional view (left) of the microfluidic channel showing the hydrogel deposition in the trap and in the channel’s corners and 3D rendering (right), taken with an in-house light sheet fluorescence microscopy set-up. h Representative images of vascular spheroid/organoid cultured in fibrin (left), and hiPSC-derived lung organoids cultured in Matrigel, showing efficient trapping and robust growth over 2 weeks on-chip (right).” Reproduced from Quintard, C., Tubbs, E., Jonsson, G. et al. A microfluidic platform integrating functional vascularized organoids-on-chip. Nat Commun 15, 1452 (2024). under a CC BY 4.0 DEED Attribution 4.0 International license.
The microfluidic chips were fabricated using Cyclic Olefin Copolymer (COC), chosen for its low autofluorescence, strong chemical resistance, and low drug absorption qualities. The microfluidic patterns were machined directly onto a COC sheet using high-precision milling equipment. The design featured 10 identical microfluidic circuits on a chip measuring 84 mm by 54 mm. For experiments with mesenchymal spheroids, the channels had dimensions of 400 µm x 400 µm, while for blood vessel organoids, the channels were 800 µm x 800 µm. These microfluidic channels were sealed with an optical adhesive film
The microfluidic setup began by introducing a non-polymerized hydrogel embedding spheroids/organoids and endothelial cells into the channel, followed by air and then growth medium. A serpentine loop within the channel, bypassed by a U-cup shaped microchannel, acted as a cell aggregate trap. The hydraulic resistance of the trap when unoccupied was less than that of the serpentine loop, guiding spheroids/organoids into the trap by flow preference. The seeding process involved gently extracting a spheroid/organoid from a 96-well plate, mixing it into the hydrogel with thrombin, and then placing this mixture into the system’s reservoir. A syringe pump, set to a withdrawal mode at a flow rate of 300 µl/min, allowed the spheroid/organoid to progress through the channel and be captured by the U-cup microchamber. Subsequent introduction of air positioned the hydrogel before it solidified, securing the spheroid/organoid within the U-cup and lining the channel corners.
The quantitative data from this study not only demonstrate the platform’s potential for creating more physiologically relevant tissue models but also highlight its applicability across various fields of biomedical research. The ability to efficiently vascularize organoids on-chip opens up new possibilities for studying disease mechanisms, testing pharmacological agents, and exploring regenerative medicine strategies with unprecedented detail and relevance.
“Our platform now allows us to explore diverse topics, such as organoid lifespan enhancement through vascularization, exposure to drugs, nucleic acids or metabolic stress. The device we have developed also offers the flexibility to vascularize other types of organoids, spheroids, tumoroids, or human tissue explants, as exclaimed in our study by improved glucose responsiveness of islet spheroids. “, the authors concluded.
Figures are reproduced from Quintard, C., Tubbs, E., Jonsson, G. et al. A microfluidic platform integrating functional vascularized organoids-on-chip. Nat Commun 15, 1452 (2024). https://doi.org/10.1038/s41467-024-45710-4 under a CC BY 4.0 DEED Attribution 4.0 International license.
Read the original article: A microfluidic platform integrating functional vascularized organoids-on-chip
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


