11 Oct Unraveling the Impacts of Flow Fluctuations on Biofilm Development with Microfluidics
In the intricate world of microfluidics, researchers are constantly unveiling new insights that bridge the gap between theoretical predictions and real-world applications. A recent study published in Nature delves deep into the impacts of flow fluctuations on the development of Pseudomonas putida biofilms, offering groundbreaking insights that could revolutionize our approach to controlling and exploiting biofilms.
“Here, we reveal the effects of flow fluctuations on the development of Pseudomonas putida biofilms through systematic microfluidic experiments and the development of a theoretical model. Our experimental results showed that biofilm growth under fluctuating flow conditions followed three phases: lag, exponential, and fluctuation phases. In contrast, biofilm growth under steady-flow conditions followed four phases: lag, exponential, stationary, and decline phases.“, the authors explained.
The Microfluidic Investigation
Biofilms are integral in various applications, including wastewater treatment and bioremediation. However, understanding their formation and growth dynamics, especially under fluctuating flow conditions common in natural and engineered systems, has remained elusive. This study employs microfluidic chips and theoretical modeling to unveil the intricate dance of biofilm development under fluctuating flows.
The researchers used a microfluidic device to grow Pseudomonas putida biofilms under various flow conditions. They meticulously observed the biofilms’ development under fluctuating and steady flow conditions, revealing distinct growth phases for each. Under fluctuating flow, the biofilm growth exhibited three phases: lag, exponential, and fluctuation, while steady flow conditions led to an additional decline phase.
The study utilized a straight microfluidic channel made of polydimethylsiloxane (PDMS), a gas-permeable material, bonded to a glass coverslip. Pseudomonas putida biofilms were grown under four flow conditions, including steady flow and fluctuating flows at low, medium, and high frequencies, all maintaining the same mean shear stress. To ensure that flow fluctuations did not impact biofilm development by altering oxygen and nutrient concentrations, the researchers calculated the diffusion and replenishment timescales of oxygen and nutrients into the microchannel. They found these timescales to be much smaller than the shortest time interval of the fluctuating flows, confirming that oxygen and nutrients remained abundant during the experiments.
The researchers observed two types of biofilms: membranelike thin-film structures on the channel’s sidewalls and heterogeneous ripple-like biofilms on the top PDMS surfaces. The biofilm thickness and areal coverage were calculated as functions of growth time, offering quantitative insights into the impacts of flow fluctuations on biofilm growth. Low-frequency flow fluctuations were found to promote biofilm growth, while high-frequency fluctuations inhibited it. These contradictory impacts were attributed to the adjustment time needed for the biofilm to grow after the shear stress changed from high to low.
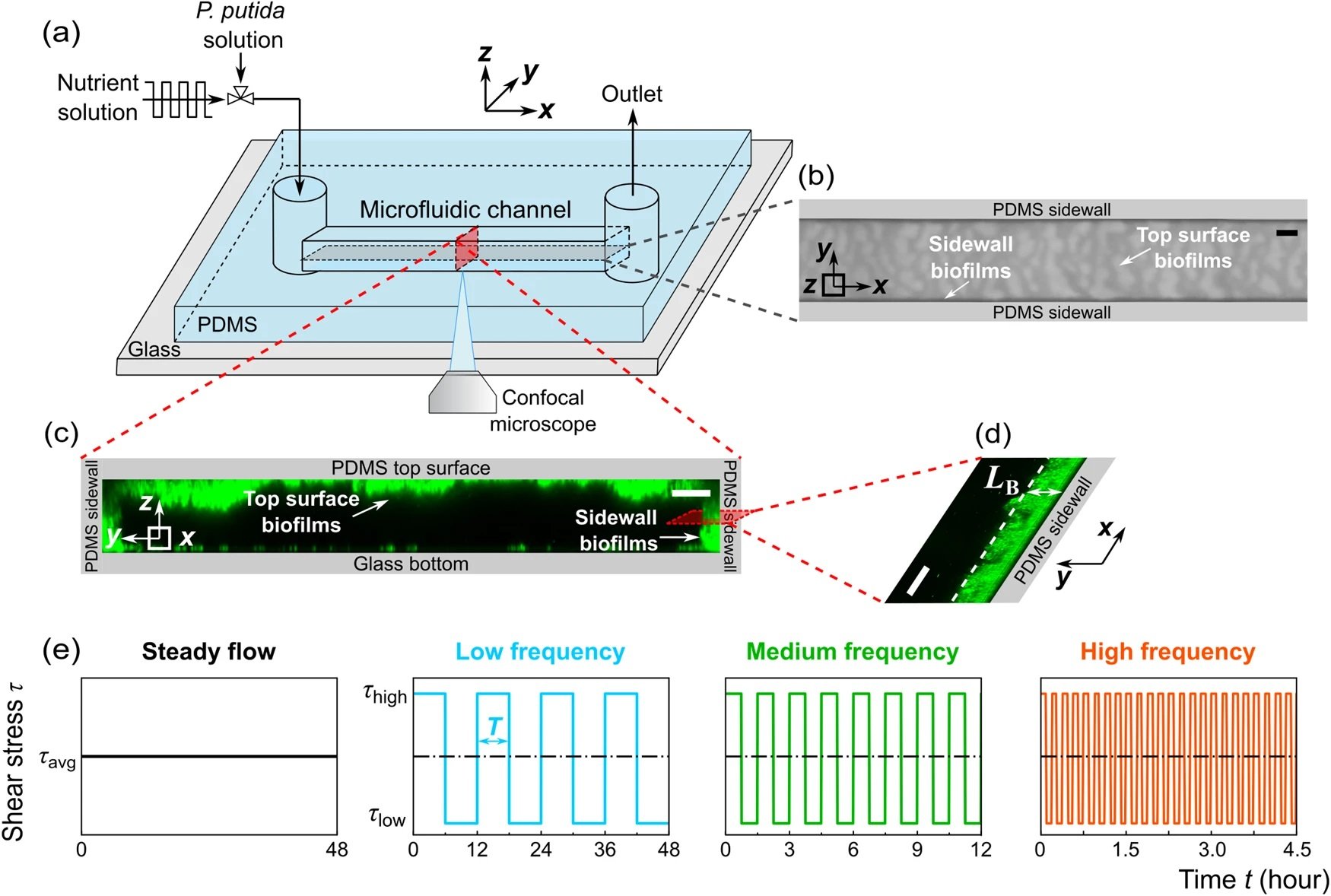
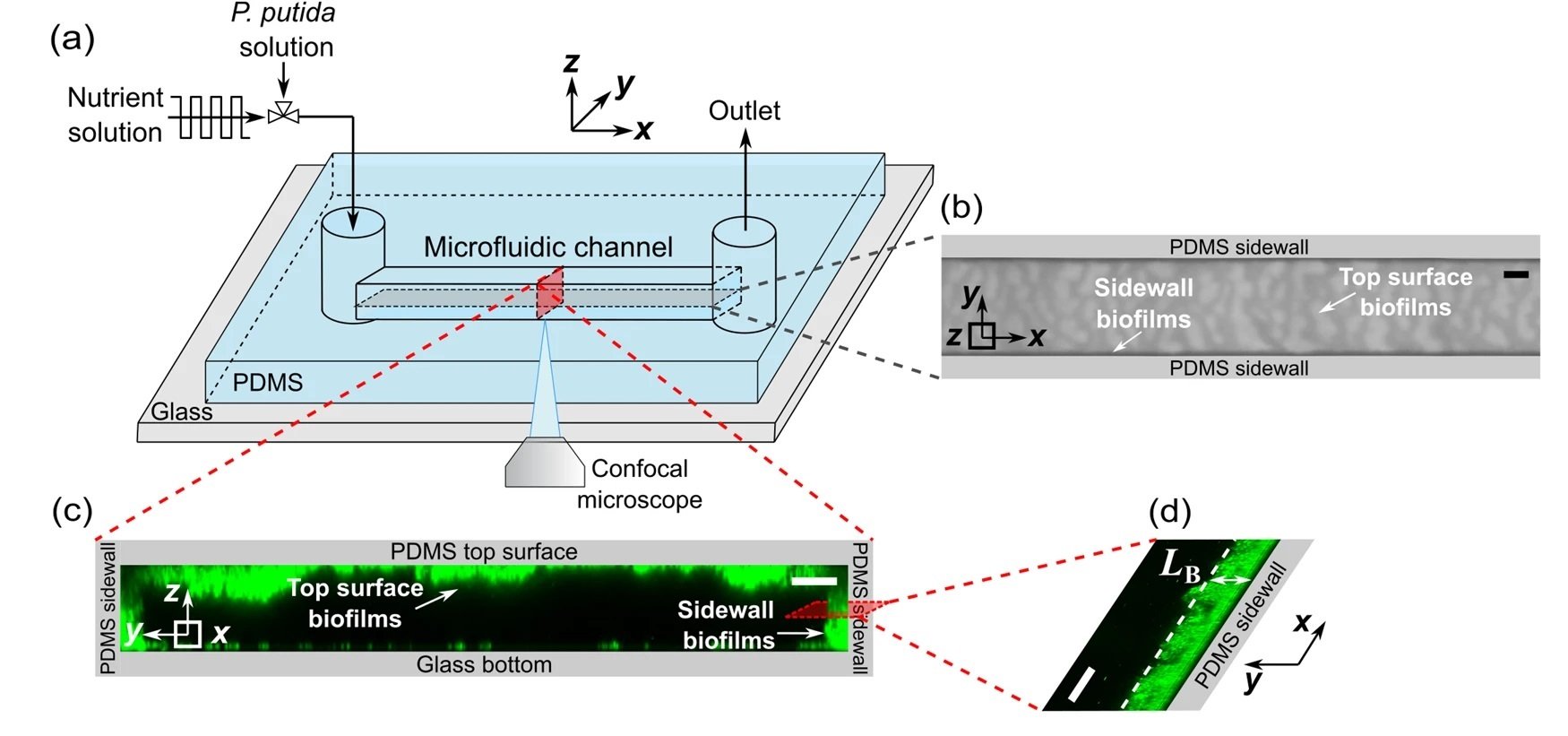
“a Schematic diagram of the experimental setup to observe the development of biofilms in a microfluidic channel. The x, y, and z axes denote the flow direction, lateral direction, and vertical direction, respectively. b Microscopic image of biofilms, using the transmitted detector (TD) function of the confocal microscope, in the horizontal cross-sectional plane of the channel, indicated by the gray color in (a). The scale bar is 100 μm. The biofilm-related parameters were measured from the TD images. c 3D images of the biofilms stained by a nucleic acid staining dye (SYTO-9), using the fluorescence detector of the confocal microscope, in the red vertical y–z plane in (a). The scale bar is 25 μm. d A zoomed-in confocal image showing biofilms on the sidewalls of the channel in the x–y plane. LB denotes the average biofilm thickness on the sidewall of the channel. The scale bar is 25 μm. e The nutrient solution was injected into the microfluidic channel for 48 h under four flow conditions: steady-flow (black line, frequency f = 0 Hz), low-frequency fluctuating flow (cyan line, frequency f = 2×10-5 Hz, the duration for low or high shear stress TLF is 6 h), medium-frequency fluctuating flow (green line, f = 2×10-4 Hz, TMF = 45 min), and high-frequency fluctuating flow (orange line, f = 1 × 10−3 Hz, THF = 5.6 min). The average, low, and high shear stress is τavg = 3.5 Pa, τlow = 0.05 Pa, and τhigh = 6.9 Pa, respectively.” Reproduced from Del Campo Fonseca, A., Glück, C., Droux, J. et al. Ultrasound trapping and navigation of microrobots in the mouse brain vasculature. Nat Commun 14, 5889 (2023). under Creative Commons Attribution 4.0 International License.
Analytical Approach
The team employed Fast Fourier Transform (FFT) analysis to determine the dominant frequency of biofilm thickness variations during the fluctuation phase, confirming that these variations were due to the time-varying shear stress. They also calculated the flow-induced pressure in the channel using the Hagen-Poiseuille equation and concluded that the change in biofilm thickness during flow fluctuations was not due to this pressure but rather to shear-induced biofilm detachment.
Theoretical Model
A theoretical model was developed to explain the observed biofilm growth under fluctuating flow conditions. This model is instrumental in offering insights into the mechanisms underlying biofilm development under such conditions, paving the way for designing strategies to control biofilm formation in various natural and engineered systems.
Implications and Future Directions
This microfluidic investigation into the impacts of flow fluctuations on Pseudomonas putida biofilm development is a significant leap forward in our understanding of biofilm dynamics. The combination of microfluidic experiments and theoretical modeling offers a comprehensive view of the intricate processes governing biofilm growth under varying flow conditions.
The insights gleaned from this study are not just academically intriguing but hold practical implications for enhancing the efficacy of biofilm-based bioremediation projects, wastewater treatment, and understanding medical device-related infections. In the realm of microfluidics, every discovery propels us a step closer to harnessing the full potential of biofilms, and this study is undoubtedly a giant leap in that direction.
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds.
Figures are reproduced from Wei, G., Yang, J.Q. Microfluidic investigation of the impacts of flow fluctuations on the development of Pseudomonas putida biofilms. npj Biofilms Microbiomes 9, 73 (2023). https://doi.org/10.1038/s41522-023-00442-z under a Creative Commons Attribution 4.0 International License)
Read the original article: Microfluidic investigation of the impacts of flow fluctuations on the development of Pseudomonas putida biofilms


