02 Oct Navigating the Brain’s Intricate Pathways: Ultrasound-Activated Microrobots Meet Microfluidics
In the ever-evolving field of microfluidics, the integration of innovative technologies continues to redefine the boundaries of what is achievable. One of the most recent breakthroughs, as detailed in a research article published in Nature Communications, unveils the successful navigation of ultrasound-activated microrobots within the intricate vasculature of the mouse brain. This pioneering study not only underscores the versatility of microfluidic devices but also opens new frontiers in targeted drug delivery and treatment of neurodegenerative diseases.
“Our microrobots consist of lipid-shelled microbubbles that autonomously aggregate and propel under ultrasound irradiation. We investigate their capacities in vitro within microfluidic-based vasculatures and in vivo within vessels of a living mouse brain.“, the authors explained.
Unraveling the Complex Brain Vasculature with Microfluidics
The brain, with its complex and delicate structure, has always posed significant challenges to the medical field, especially in the delivery of drugs to specific regions. Traditional methods often fall short in precision and effectiveness. However, the advent of microfluidic chips and advanced microfabrication techniques, characterized by its precision, efficiency, and minimal invasiveness, is changing the narrative.
In the highlighted study, researchers ingeniously employed a microfluidic device to investigate the behavior of lipid-shelled microbubbles – the microrobots – in vitro. These microrobots, activated by ultrasound, showcased an unprecedented ability to navigate the brain’s vasculature, moving against blood flows of up to 10 mm/s. The integration of microfabrication and ultrasound technology enabled real-time visualization and control, marking a significant stride in in vivo brain research.

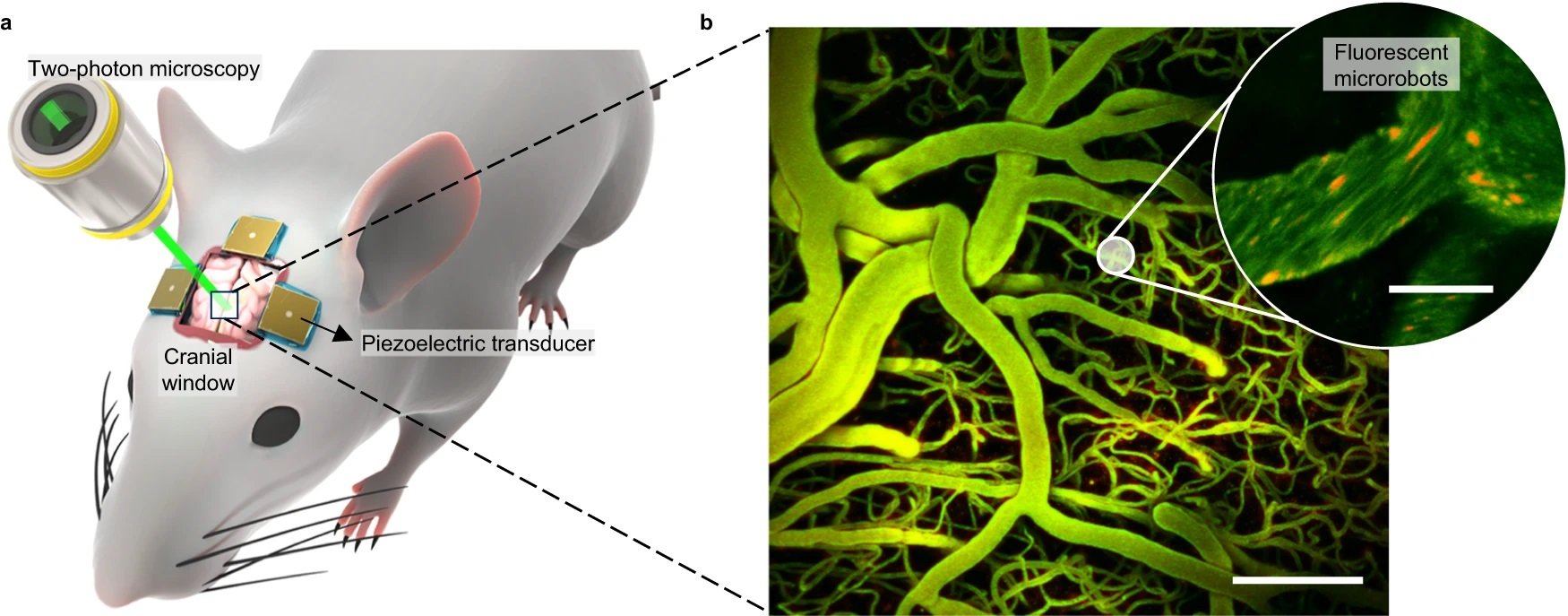
“a Setup for in vivo studies. Optical access to the brain vasculature for 2P microscopy is provided by a cranial window. Piezoelectric transducers are attached to the skull and introduce acoustic waves directed towards the brain. b Brain vasculature as visualized by 2P microscopy (z-stack projection). Scale bar is 100 µm. In the inset, fluorescent microbubble-based swarms that will form the microrobots are seen in red. A total sample of 200 microbubble clusters and 39 blood vessels were visualized with this technique during the experiments. For a detailed explanation of the image processing, see “Methods”. Scale bar is 10 µm.” Reproduced from Del Campo Fonseca, A., Glück, C., Droux, J. et al. Ultrasound trapping and navigation of microrobots in the mouse brain vasculature. Nat Commun 14, 5889 (2023). under Creative Commons Attribution 4.0 International License.
Microfluidic Devices at the Forefront
The microfluidic chip used in the study was instrumental in mimicking the brain’s vasculature, offering insights into the microrobots’ behavior and navigation capabilities. The precision and control afforded by microfluidic technology were pivotal in observing the microrobots’ self-assembly and propulsion under ultrasound irradiation.
The synergy between microfluidics and ultrasound technology not only enhanced the visualization of microrobots but also ensured their efficient propulsion. The microrobots, upon exposure to ultrasound, self-assembled and got trapped at the walls of blood vessels, translating along the walls even against blood flow.
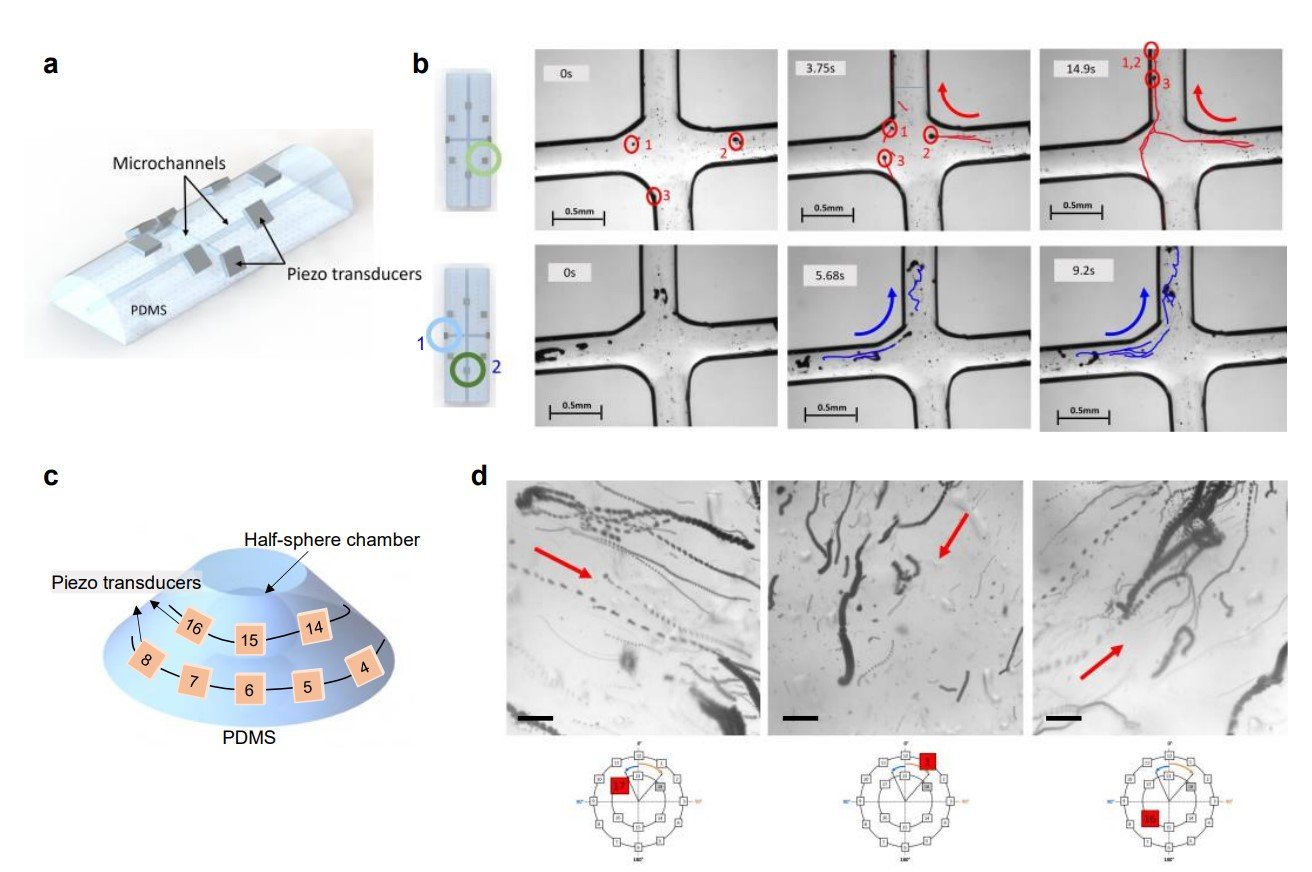
“Formation and navigation of microswarms along defined trajectories with a combination of single piezoelectric transducers. a. Schematic of the experimental setup used for the experiment. 8 transducers were attached to a PDMS device. The microfluidic device contained a cross design mimicking an intersection between two vessels. b. Image sequence of microbubble behavior upon acoustic excitation at 242kHz and 3VPP of transducers. Transducers were activated in a sequential way, as marked by labels 1 and 2 in the left image. As seen in the various time frames, microbubbles assemble into a swarm, and they move in the direction of wave propagation. The swarms can move into the desired branches upon activation of the correct transducers. Similar behaviors were achieved in at least 5 independent experiments. Scale bar is 500 µm. c. Design A. Schematic of the cone frustum PDMS experimental set-up used for experiments. 18 transducers were attached to the PDMS device distributed in two rows. Inside the device we fabricated a half-sphere hollow chamber where we later injected a microbubble containing solution. d. Each image consists of a stack of images during acoustic activation. In black we see the microbubbles and in a red arrow we show the trajectory of microbubbles navigation when one transducer is activated. Below each image we have marked in red the activated transducer. Each transducer was activated 3 times getting reproducible results. Scale bar is 100 µm. ”. Scale bar is 10 µm.” Reproduced from Del Campo Fonseca, A., Glück, C., Droux, J. et al. Ultrasound trapping and navigation of microrobots in the mouse brain vasculature. Nat Commun 14, 5889 (2023). under Creative Commons Attribution 4.0 International License.
A Closer Look at the Experimental Process
Microbubble Formation and Ultrasound Activation
The core of this innovative research lies in the meticulous formation and manipulation of lipid-shelled microbubbles. These microbubbles, serving as microrobots, are engineered to have a specific resonance frequency that responds to ultrasound waves. The microbubbles are injected into the bloodstream and, upon ultrasound activation, aggregate and adhere to the blood vessel walls. This adhesion is a crucial step, allowing for the precise navigation and control of these microrobots within the vasculature.
Microfluidic Device and In Vitro Testing
The researchers employed a custom-designed microfluidic chip, a masterpiece of microfabrication technology, to simulate the intricate and complex vasculature of the mouse brain. This microfluidic device allowed for the in-depth analysis of the microrobots’ behavior, offering real-time insights into their navigation capabilities and responses to various flow rates and ultrasound frequencies.
In the in vitro tests, the microfluidic chip was instrumental in observing the self-assembly of microbubbles and their propulsion under ultrasound irradiation. The microfluidic environment, characterized by its precision and control, provided a conducive setting for monitoring the microrobots’ robustness against high flow rates and their adaptability to complex three-dimensional cerebral capillary networks.
In Vivo Experiments and Two-Photon Microscopy
The in vivo experiments involved injecting fluorescent microbubbles into the mouse bloodstream. Up to four piezoelectric transducers, positioned on the sides of a cranial window, were used for ultrasound stimulation. The combination of ultrasound and two-photon microscopy enabled the researchers to achieve real-time visualization of the microrobots navigating the living mouse brain.
The microrobots demonstrated an unprecedented ability to move against blood flows of up to 10 mm/s, underscoring their potential for precise local drug delivery in hard-to-reach brain regions. The real-time visualization facilitated by the integration of ultrasound technology and two-photon microscopy was pivotal in monitoring and controlling the microrobots’ navigation.
Implications and Future Directions
This groundbreaking study underscores the pivotal role of microfluidic devices in advancing biomedical research. The precision, control, and versatility of microfluidics are unlocking new possibilities, especially in navigating the complex and delicate structures of the brain.
As we continue to witness the convergence of microfluidics with other innovative technologies like ultrasound and two-photon microscopy, the prospects for targeted drug delivery, non-invasive treatments, and comprehensive understanding of the brain’s physiology are becoming increasingly tangible.
In conclusion, the intersection of ultrasound-activated microrobots and microfluidics heralds a new era in neuroscience and targeted drug delivery. The precision and control intrinsic to microfluidic devices, coupled with the real-time visualization and manipulation capabilities of ultrasound technology, are set to revolutionize the treatment of cerebrovascular and neurodegenerative diseases. The future of microfluidics in biomedical research is not just promising; it is here, unfolding with every innovative study and every successful integration of multidisciplinary technologies.
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds.
Figures are reproduced from Del Campo Fonseca, A., Glück, C., Droux, J. et al. Ultrasound trapping and navigation of microrobots in the mouse brain vasculature. Nat Commun 14, 5889 (2023). https://doi.org/10.1038/s41467-023-41557-3 under a Creative Commons Attribution 4.0 International License)
Read the original article: Ultrasound trapping and navigation of microrobots in the mouse brain vasculature


