01 Sep Exploring Bacterial Behavior: Advancing Microbial Research with Microfluidics and Single-Cell Sequencing
The world of microorganisms is a complex and diverse realm that plays a vital role in various ecological, medical, and industrial processes. Understanding the intricate interactions and behaviors of different bacterial species can offer valuable insights into disease mechanisms, environmental dynamics, and even biotechnological applications. A groundbreaking study titled “Exploring Bacterial Diversity through Single-Cell Sequencing” utilized microfluidics technology and dives deep into this microscopic world using innovative techniques and cutting-edge technologies.
The research article, authored by an international team of scientists, presents a comprehensive exploration of bacterial diversity through a technique known as microfluidic single-cell sequencing. This approach allows researchers to analyze individual bacterial cells, uncovering their genetic information and shedding light on their unique characteristics and functions.
“In this study, we developed a droplet-based, high-throughput, and high-sensitivity single-microbe RNA-seq method, named smRandom-seq, with the pipeline of bacteria preparation, reactions in situ, droplet barcoding, and library preparation. We provided sufficient and systematic evidence to prove the efficiency and technical reproducibility of smRandom-seq and validated the performance of smRandom-seq by practical application.“, the authors explained.
The study’s workflow was meticulously designed to capture the diversity and intricacies of bacterial behavior. The researchers began by cultivating various bacterial strains, including Escherichia coli, Bacillus subtilis, Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus. These cultures were grown and sampled under controlled conditions, providing the basis for subsequent experiments.

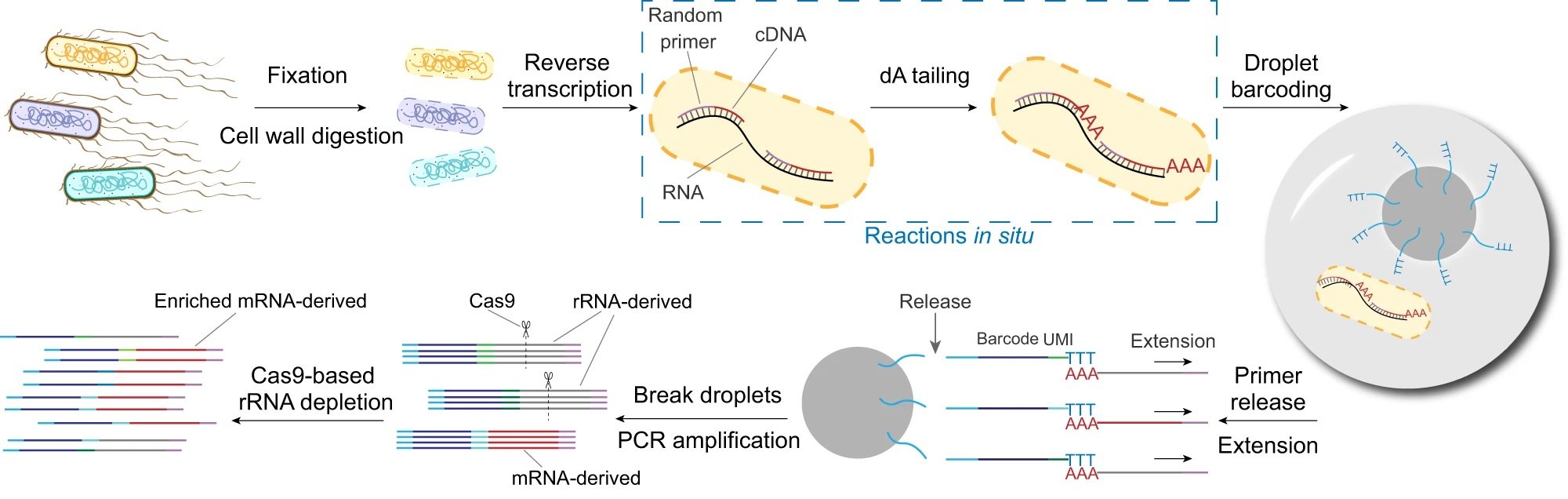
“The workflow of smRandom-seq for microbe samples includes fixation, cell wall digestion, reverse transcription, dA tailing, droplet barcoding, primers releasing and extension, droplets breaking and PCR amplification, and Cas9-based rRNA depletion and sequencing. Blue dashed box: the two in situ reactions, including reverse transcription and dA tailing. AAA: dA tail in the 3’ of cDNA, TTT: poly(dT) in the barcoded primers.” Reproduced from Xu, Z., Wang, Y., Sheng, K. et al. Droplet-based high-throughput single microbe RNA sequencing by smRandom-seq. Nat Commun 14, 5130 (2023). under Creative Commons Attribution 4.0 International License.
One of the study’s pivotal components was the utilization of microfluidic devices. These miniature platforms enabled the synthesis of hydrogel barcoded beads and the encapsulation of individual bacterial cells. In the hydrogel bead synthesis process, microfluidic channels with specific dimensions were used to emulsify and polymerize an acrylamide-primer mix, resulting in hydrogel barcoded beads. These beads were then combined with unique barcoded primers, which were subsequently used to label and track individual bacterial cells. This technique allowed researchers to label each bacterium with a unique barcode, ensuring precise tracking during analysis.
The microfluidic chips were custom-designed for both hydrogel bead synthesis and single bacterium barcoding, facilitating intricate manipulations on a microscopic scale. Furthermore, the microfluidic devices allowed for the encapsulation of single bacterial cells alongside hydrogel barcoded beads. This allowed researchers to study the gene expression profiles of individual bacteria in a controlled environment, facilitating the accurate identification of marker genes and the characterization of different bacterial clusters.
Following the sequencing of the barcoded bacterial samples, advanced bioinformatics tools were employed for data processing and analysis. The team employed normalization, scaling, and dimensionality reduction techniques to derive meaningful insights from the extensive genetic data obtained. This enabled the identification of distinct clusters, differential expression patterns, and functional enrichment.
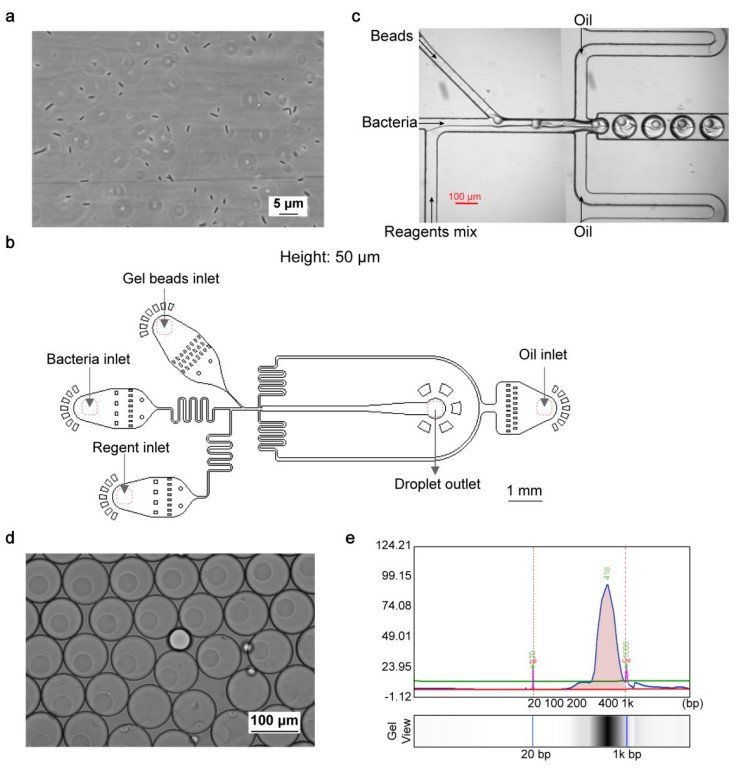
“a, Image of fixed E. coli after reverse transcription and dA tailing. Scale bar: 5 μm. n= 4 independent experiments. b, Design of the device for cell, bead and mix reagents encapsulation. Height: 50 μm. c, Image of microfluidic barcoding device. Scale bar: 100 μm. d, Image of encapsulated droplets. Scale bar: 100 μm. e, Electropherogram of amplicon from barcoded single bacterium cDNAs. Lower marker: 20 bp; upper marker: 1k bp.” Reproduced from Xu, Z., Wang, Y., Sheng, K. et al. Droplet-based high-throughput single microbe RNA sequencing by smRandom-seq. Nat Commun 14, 5130 (2023). under Creative Commons Attribution 4.0 International License.
The study’s findings have far-reaching implications for various scientific domains. By deciphering the gene expression profiles of individual bacterial cells, researchers gained insights into their distinct characteristics and functions. This knowledge can enhance our understanding of microbial communities in environments such as the human gut and industrial settings, leading to informed decisions in medicine, biotechnology, and more.
“Our study provides a way to tease out how individual bacterium adapts and interacts with each other under environmental stress. We can then efficiently predict which subpopulations will evolve resistance and survive to impact health, as well as figure out the mechanisms of bacterial resistance and persistence, which would be valuable for precision diagnosis and treatment of bacterial infection. Moreover, combined with the Combi-Seq49 which uses a combinatorial DNA barcoding approach to encode treatment conditions in droplets, smRandom-seq would probably allow monitoring the bacterial transcriptomic changes at a single bacterium level for high-throughput drug screening. “, the authors explained.
Remember to check our blog for more exciting research highlights and stay up to date with the latest advancements in science and technology!
Figures are reproduced from Xu, Z., Wang, Y., Sheng, K. et al. Droplet-based high-throughput single microbe RNA sequencing by smRandom-seq. Nat Commun 14, 5130 (2023). https://doi.org/10.1038/s41467-023-40137-9 under a Creative Commons Attribution 4.0 International License)
Read the original article: Droplet-based high-throughput single microbe RNA sequencing by smRandom-seq


