16 Apr A Novel Microfluidic Tool for Studying Neurocutaneous Diseases and Drug Screening
Understanding the interactions among various cells and neighboring microenvironmental components in the skin is essential for research and development and industrial applications, but challenges have existed in reconstituting 3D structures of cutaneous innervation in vitro and developing analysis tools in a cell-type-specific manner. Researchers at Korea University have employed microfluidics to mimic the innervated epidermis of human skin in vitro, providing a platform for studying neurocutaneous diseases and drug screening with a level of complexity not found in conventional skin models. The developed microfluidic chip provides an alternative to animals and allows for approximations of various human skin diseases and side effects of other diseases on the skin.
“Our co-culture model recapitulates a more organized basal-suprabasal stratification, enhanced barrier function, and physiologically relevant anatomical innervation and demonstrated the feasibility of in situ imaging and functional analysis in a cell-type-specific manner, thereby improving the structural and functional limitations of previous coculture models. This system has the potential as an improved surrogate model and platform for biomedical and pharmaceutical research. “, the authors explained.
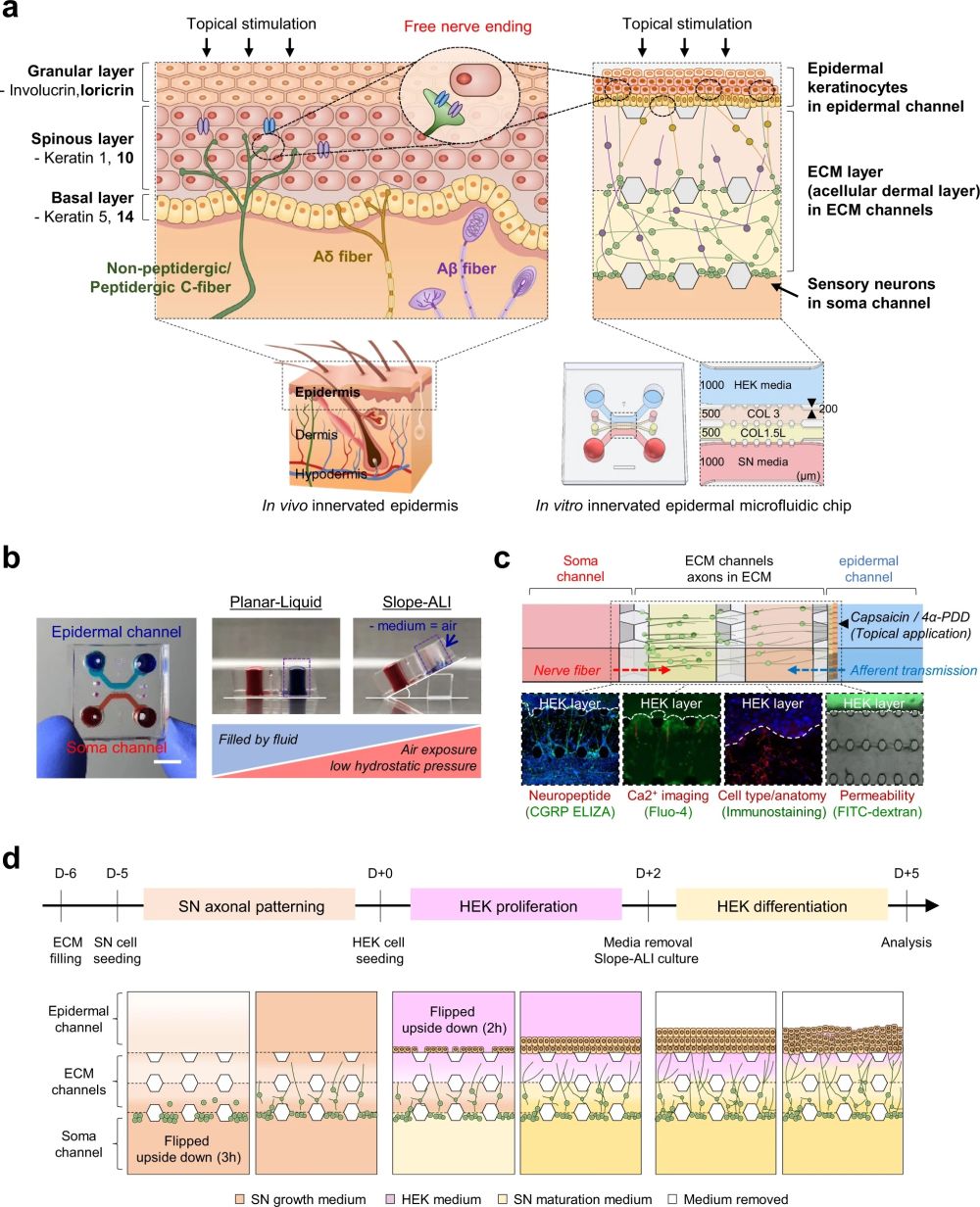
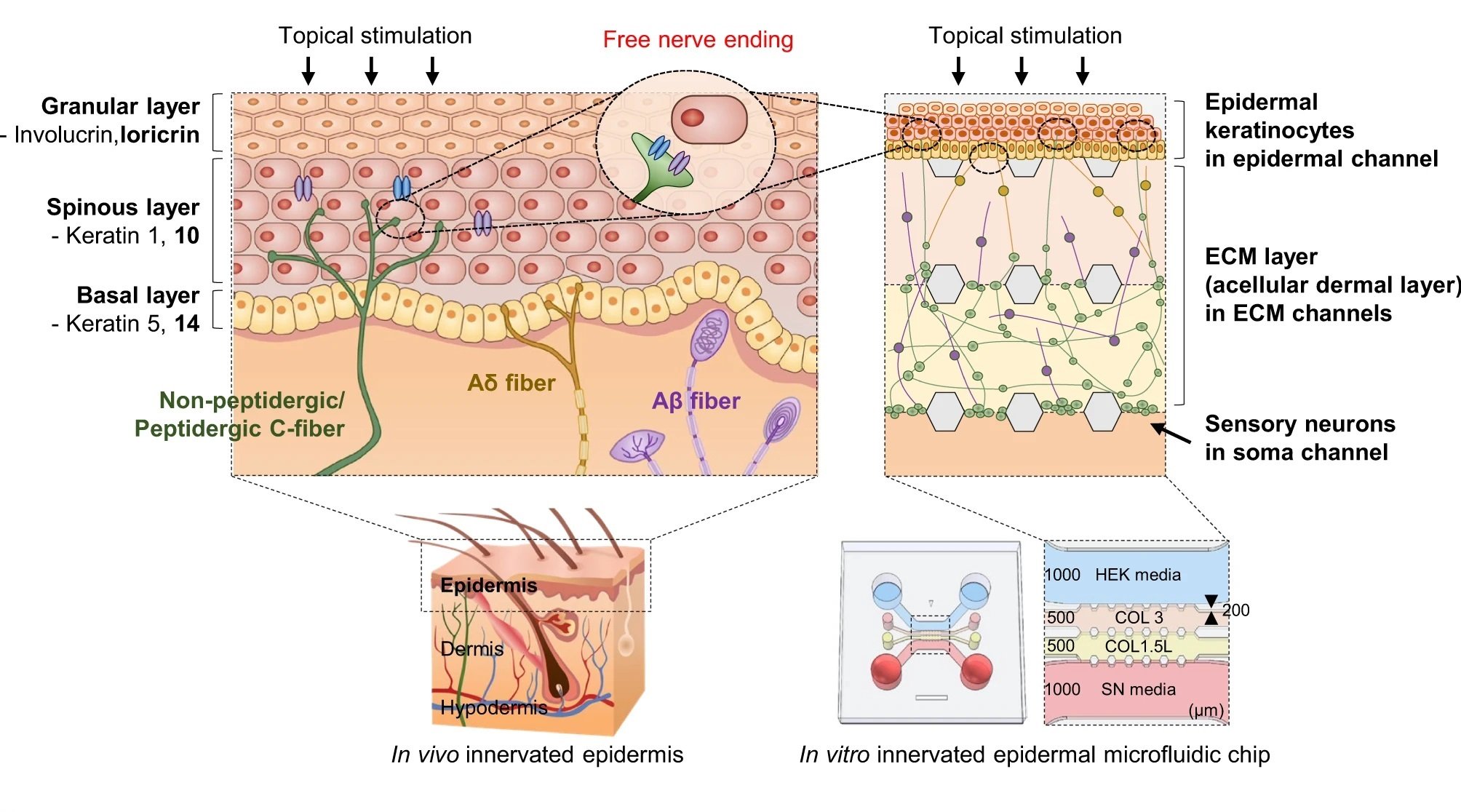
“a Schematic illustration and design of human skin anatomy (left) and the innervated epidermal chip to coculture sensory neurons and keratinocytes (right). Schematic design of the innervated epidermal chip compartments (right lower). HEK; human keratinocyte, SN; sensory neuron, COL 3; collagen I at 3 mg/ml concentration, COL 1.5 L; collagen I at 1.5 mg/mL with 10% laminin, Scale unit; μm. b Top view of the microfluidic chip (left) and experimental concept of slope-based air-liquid interface (ALI) method for epidermal development (right, longitudinal vertical section view). Each cell channel was marked with a different color dye. c Cell-type-specific assays for the innervated epidermal chip. d Experimental workflow of cell seeding and culture for generating the innervated epidermal chip.” Reproduced Ahn, J., Ohk, K., Won, J. et al. Modeling of three-dimensional innervated epidermal like-layer in a microfluidic chip-based coculture system. Nat Commun 14, 1488 (2023). under Creative Commons Attribution 4.0 International License.
The chip was fabricated using microfluidic fabrication techniques and a SU-8 patterned wafer via soft lithography using polydimethylsiloxane (PDMS). The device was produced by bonding the PDMS replica to a glass coverslip and filling the microchannels with a 2-mg/mL polydopamine solution (PDA) to improve cell attachment to the hydrogel. Two gel channels in the microfluidic device were then filled with two types of hydrogels, type I collagen gel solution and a collagen solution containing 10% laminin.
The proposed microfluidic device can not only serve as an alternative to animal testing but also as an approximation for various human skin diseases and side effects of other diseases on the skin. The chip allows for fluidically isolated culture of the two cell populations in distinct patterns or indirect juxtaposition on the same plane of medium and ECM hydrogel. This enables temporal and selective analysis and/or probing of specific cells, such as in situ Ca2+ response monitoring and epidermal permeability measurement for topically applied substances.
The developed chip models spatially distributed various types of sensory neurons (SN) in the acellular dermal extracellular matrix (ECM) layer and intraepidermal free nerve endings in the epidermal-like layer, recapitulating the physiological histology of human skin. The chip successfully models the interactions reciprocally contributed to the development and maturation of the innervated epidermis-like layer and afferent transmission of topical stimuli from epidermal keratinocytes to SN.
The chip also allows for the modeling of a hyperglycemic environment to understand the pathological roles and changes of innervated skin components in the development of diabetic neuropathy. The afferent transmission provides a possible mechanism for early features of acute hyperglycemia/prediabetes without electrophysiological evidence of nerve damage. It could also explain sensory dysfunction such as neuropathic pain behavior in diabetic patients despite the loss of intraepidermal nerve fibers.
In conclusion, the developed microfluidic chip provides a promising platform for studying the complex interactions among various cells and neighboring microenvironmental components in the skin and for studying neurocutaneous diseases and drug screening. With further improvements, this chip has the potential to revolutionize skin research and drug development, ultimately leading to better treatment options for skin diseases and disorders.
” Our data shows a structurally and functionally integrated innervated epidermal-like layer, recapitulating abnormal cellular interactions in a pathophysiologically relevant human setting. We hope to provide insights on the future integration of these skin models with other cell components onto microfluidic platforms as well as potential readout technologies for high-throughput drug screening “, the authors concluded.
Figures are reproduced from Ahn, J., Ohk, K., Won, J. et al. Modeling of three-dimensional innervated epidermal like-layer in a microfluidic chip-based coculture system. Nat Commun 14, 1488 (2023). https://doi.org/10.1038/s41467-023-37187-4 under Creative Commons Attribution 4.0 International License.
Read the original article: Modeling of three-dimensional innervated epidermal like-layer in a microfluidic chip-based coculture system


