08 Apr Unlocking Cellular Secrets: Fast Single-Cell Physical Phenotyping with Microfluidics
In the fight against cancer and other diseases, understanding the behavior and characteristics of individual cells within tissues is critical. Traditional methods of studying cells, such as flow cytometry and histology, are limited by their inability to capture both the physical and functional properties of cells in their native environment. In this recent article published in Nature Biomedical Engineering, researchers from Germany have developed a new method for studying single cells in tissue samples. The method, called real-time deformability cytometry (RT-DC), uses a technique called tissue dissociation to isolate single cells from tissues and then measures the deformability of the cells in real time using microfluidics and high-speed microscopy. This allows researchers to quickly and accurately analyze large numbers of cells, which could be useful in studying cancer and other diseases.
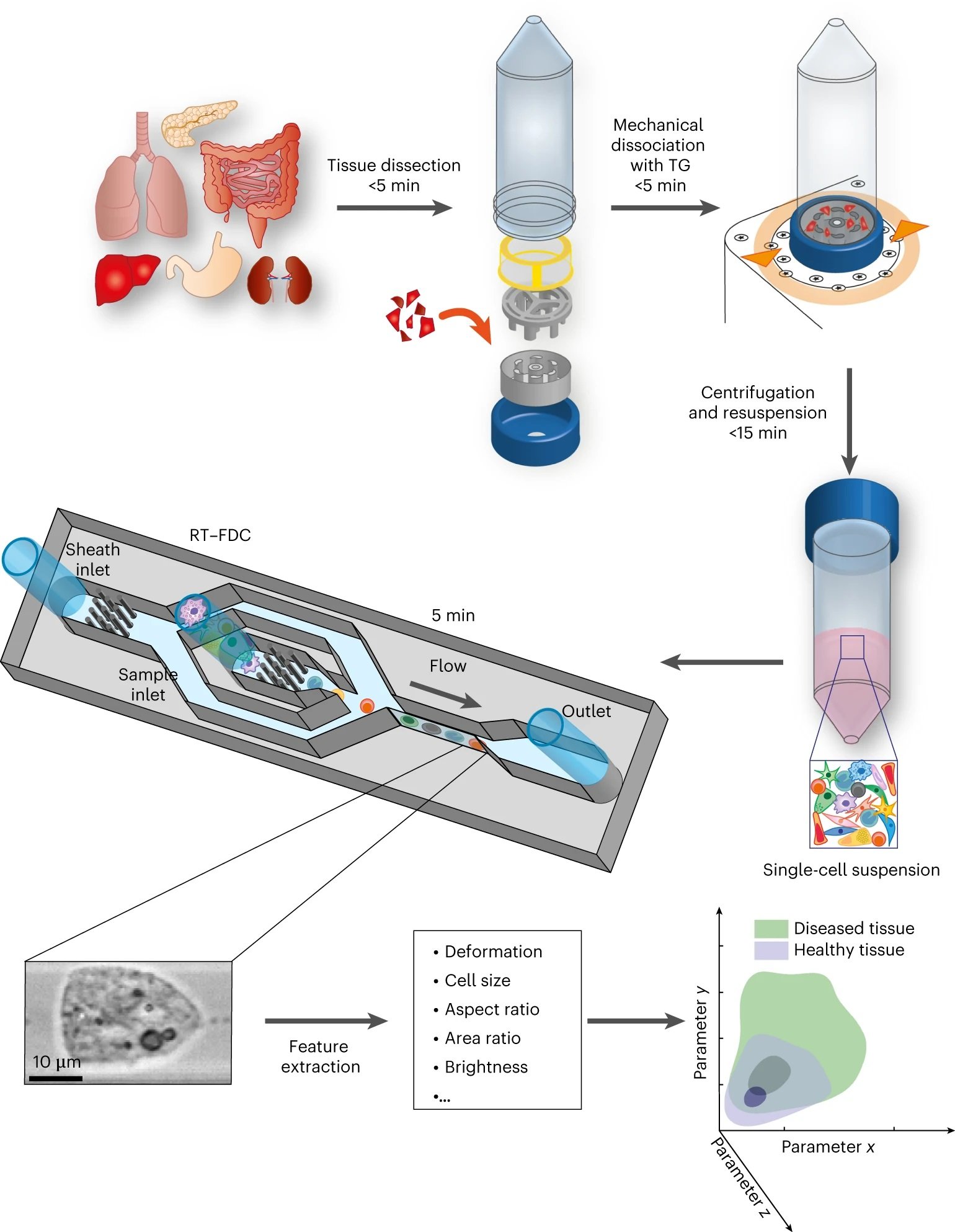
“Here we show that biopsy samples can be analysed within 30 min by sequentially assessing the physical phenotypes of singularized suspended cells dissociated from the tissues. The diagnostic method combines the enzyme-free mechanical dissociation of tissues, real-time deformability cytometry at rates of 100–1,000 cells s−1 and data analysis by unsupervised dimensionality reduction and logistic regression. “, the authors explained.

“The tissue sample is dissected in small pieces and placed into the inner rotor of the TG unit containing culture medium. Mechanical dissociation is performed by a pre-programmed, automatically executed sequence of clockwise and counter-clockwise rotations. Dissociated cells are centrifuged and resuspended in measuring buffer. The sample is loaded onto a microfluidic chip and analysed using RT–FDC. A brightfield image of every single one of typically 10,000 cells is captured. Various features are extracted from the images, which are used for multi-dimensional analysis. In total, the procedure from tissue to result takes less than 30 min.” Reproduced from Soteriou, D., Kubánková, M., Schweitzer, C. et al. Rapid single-cell physical phenotyping of mechanically dissociated tissue biopsies. Nat. Biomed. Eng (2023) under Creative Commons Attribution 4.0 International License.
In their study, they utilized a microfluidic device for our RT-FDC measurements. The chip was designed and microfabricated using standard microfluidic fabrication techniques using PDMS, which allowed us to create a device that was reproducible and easy to use. The microfluidic chip consisted of a sample inlet, a sheath inlet, and an outlet connected by a central channel constriction of a 20×20, 30×30, or 40×40μm square cross-section and a length of 300μm. The corresponding total flow rates used were 0.06μl/s for 20μm-1 channels, 0.12μl/s for 30μm channels, and 0.2μl/s for 40μm channels.
The microfluidic chip was used to perform RT-FDC measurements on single cells derived from both murine and human tissue samples. Their results demonstrated that the microfluidic device was highly effective at measuring a range of parameters, including morphological, mechanical, and fluorescence characteristics. In addition, the microfluidic chip allowed us to analyze cells with high throughput and accuracy, providing valuable insights into the heterogeneity and complexity of these samples.
The RT-DC provides a fast, accurate, and non-invasive method for studying single cells in tissue samples, and could be a valuable tool in cancer research and other areas of biology and medicine. Further research is needed to validate the method and explore its potential applications.
“Overall, our findings show that the physical phenotyping of cells via RT–FDC after enzyme-free mechanical tissue dissociation is a quick and simple method that can be used to diagnose pathological states in tissue biopsies. In particular, it may provide a rapid and unbiased prediction of disease state in inflammatory conditions and in malignancy. “, the authors concluded.
Figures are reproduced from Soteriou, D., Kubánková, M., Schweitzer, C. et al. Rapid single-cell physical phenotyping of mechanically dissociated tissue biopsies. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01015-3 under Creative Commons Attribution 4.0 International License.
Read the original article: Rapid single-cell physical phenotyping of mechanically dissociated tissue biopsies


