11 Mar A novel cell retention concept for reproducible microfluidic single-cell cultivation
Microfluidic devices have emerged as an innovative tool for single-cell analysis applications, but their success depends on the ability to retain cells in the cultivation chamber. Cell loss during long-term cultivation has been a significant challenge for microfluidic chips, limiting the ability to perform reproducible microfluidic single-cell studies. However, a new study has developed a novel cell retention concept that can address this challenge.
“Here, we present an easy-to-implement cell retention concept to withhold cells inside microfluidic cultivation chambers. By introducing a blocking structure into a cultivation chamber’s entrance and nearly closing it, cells can be manually pushed into the chamber during loading procedures but are unable to leave it autonomously in subsequent long-term cultivation.”, the authors explained.
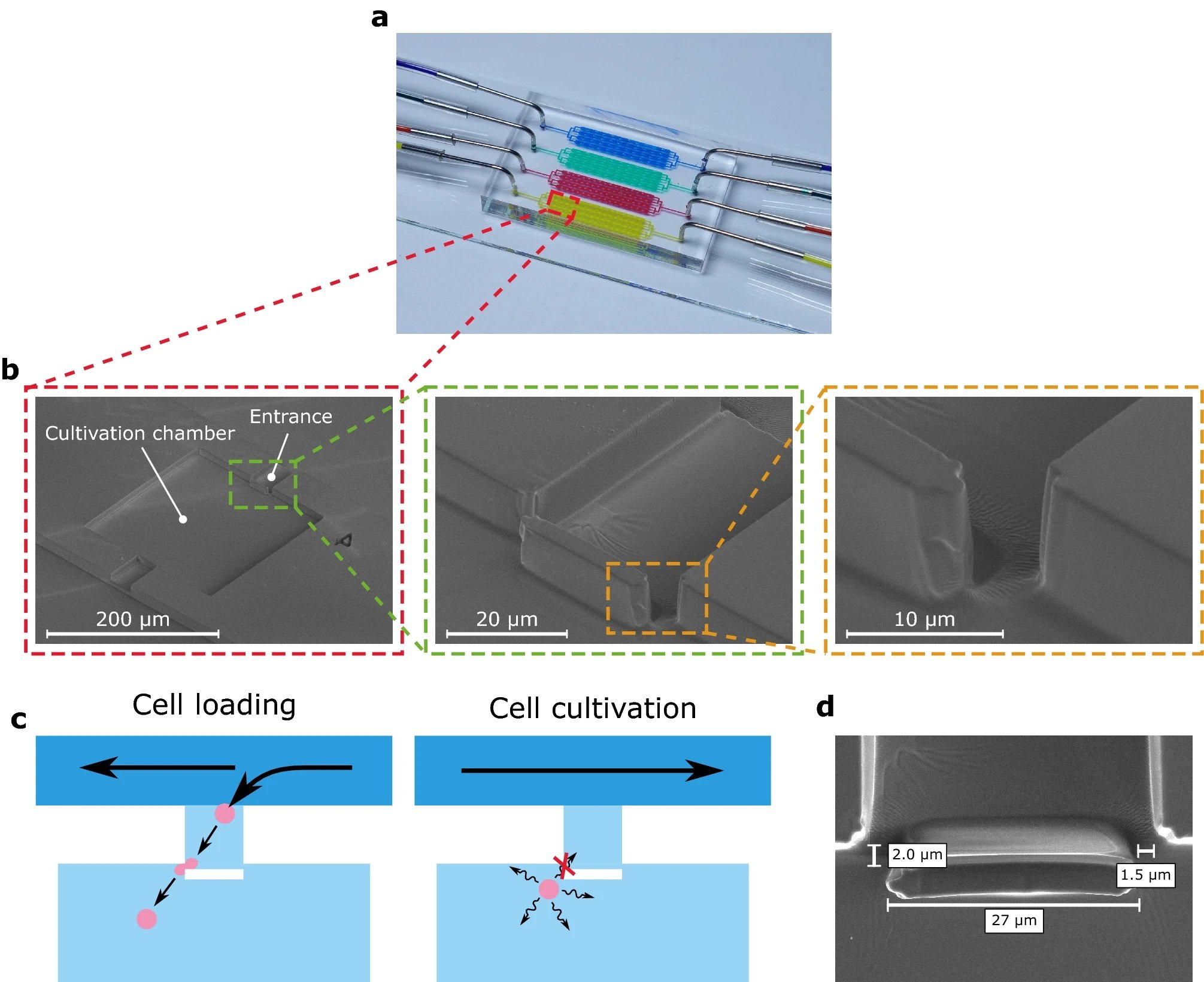
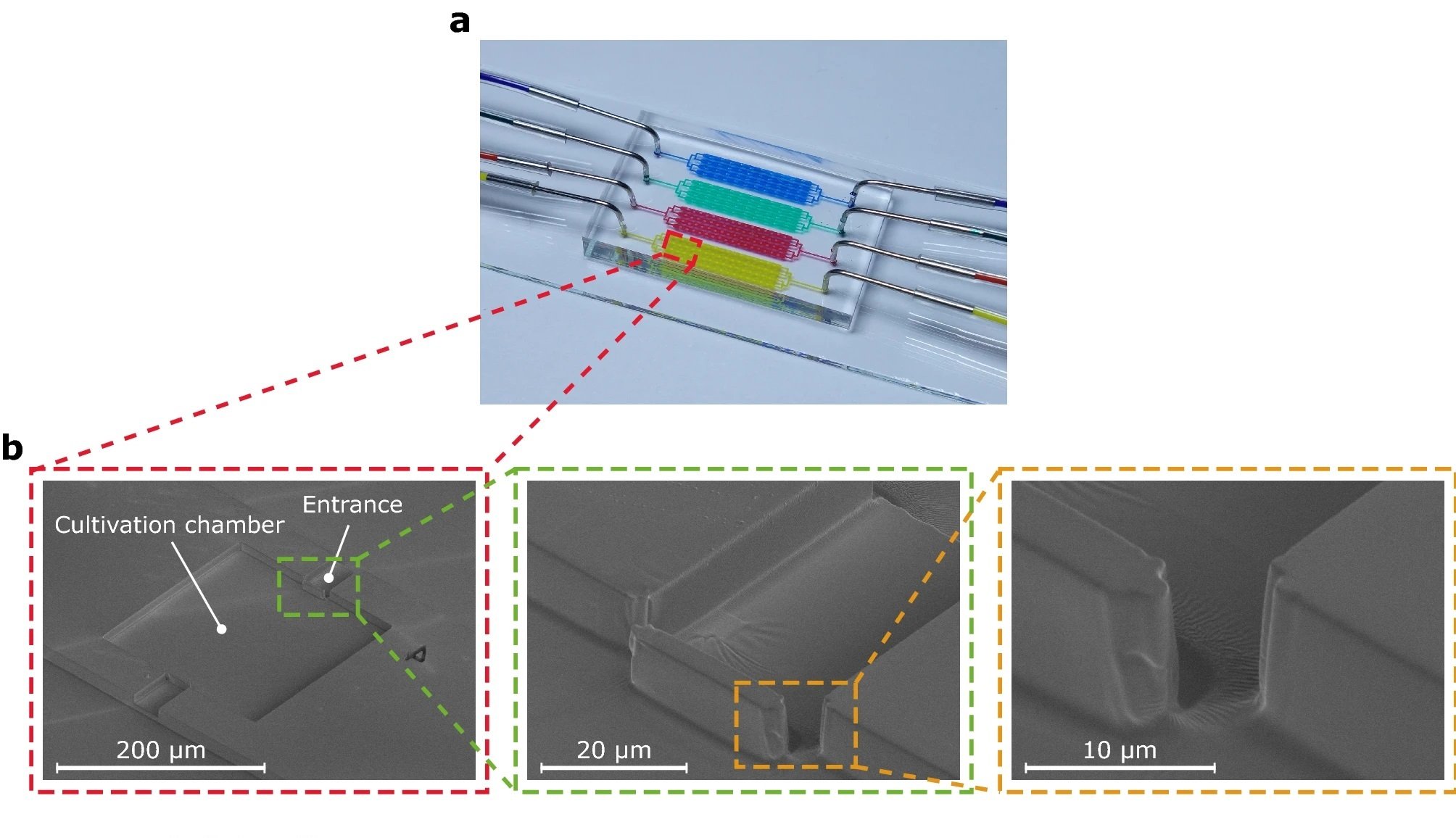
“Structure and cell retention concept of the MSCC device with enhanced cell retention for CHO suspension cell lines. (a) Microfluidic PDMS-glass-based cultivation device. (b) Scanning electron microscopy image of the microfluidic structure illustrating the devices dimensions and trapping barrier. (c) Schematic drawing of the loading procedure and cell retention concept based on a PDMS barrier that is traversable by applying pressure during cell loading but non-traversable by random cellular movement during cultivation. (d) Scanning electron microscopy image of the PDMS barrier located in the cultivation chamber’s entrance.” Reproduced from Schmitz, J., Stute, B., Täuber, S. et al. Reliable cell retention of mammalian suspension cells in microfluidic cultivation chambers. Sci Rep 13, 3857 (2023) under Creative Commons Attribution 4.0 International License.
The research team introduced a physical blocking structure into an existing microfluidic single-cell cultivation (MSCC) device to improve cell retention. This design change allowed cells to be pushed into the cultivation chamber past the barrier, but prevented them from escaping afterward. The team found that this design improved cell retention and allowed for single cells to be cultivated for multiple days without nutrient limitations. Moreover, the diffusive mass exchange was decreased, and the cultivation conditions remained constant due to steady perfusion of the device.
To validate this novel concept, the team quantified diffusive mass exchange and glucose concentration profiles inside the cultivation chamber through fluorescein trace substance experiments and computational fluid dynamics simulations. The results showed that the diffusive exchange duration increased by 100% compared to the previous MSCC device, but no limitations occurred since cellular growth was slow in comparison to the determined medium exchange duration.
The team also evaluated the cell retention capability of the new device by recording the growth of microcolonies. The results showed that the growth curves of microcolonies using the new design were more uniform, and the specific growth rate was twice as high as the previous design. The difference in cell retention was further supported by videos, showing that cells frequently left the cultivation chamber in the previous design due to their random movements, while cells in the new design only left the chamber when they were pushed out by other cells or directly divided throughout the narrow gap between the barrier and chamber wall.
Additionally, the team analyzed the growth on the single-cell level and compared it to the colony level. The results showed that the novel cell retention concept led to a consistency of single-cell and colony growth data, while the previous design led to discrepancies.
Overall, the proposed novel cell retention concept represents a significant improvement for reproducible microfluidic single-cell cultivation. The modular structure and simple way of retaining cells suggest that the principle can be transferred to other cultivation chamber-based designs and be applied for the cultivation of other organisms. The team believes that their concept can pave the way for future applications in the context of basic and biomedical single-cell research, enabling systematic studies of cellular behavior in a reproducible way without decreased significance due to constant loss of analyzed cells.
Figures are reproduced from Schmitz, J., Stute, B., Täuber, S. et al. Reliable cell retention of mammalian suspension cells in microfluidic cultivation chambers. Sci Rep 13, 3857 (2023). https://doi.org/10.1038/s41598-023-30297-5 under Creative Commons Attribution 4.0 International License.
Read the original article: Reliable cell retention of mammalian suspension cells in microfluidic cultivation chambers


