11 Aug A versatile microfluidic system for multimode stereo acoustic manipulation of single cells
Abstract
“At the single-cell level, cellular parameters, gene expression and cellular function are assayed on an individual but not population-average basis. Essential to observing and analyzing the heterogeneity and behavior of these cells/clusters is the ability to prepare and manipulate individuals. Here, we demonstrate a versatile microsystem, a stereo acoustic streaming tunnel, which is triggered by ultrahigh-frequency bulk acoustic waves and highly confined by a microchannel. We thoroughly analyze the generation and features of stereo acoustic streaming to develop a virtual tunnel for observation, pretreatment and analysis of cells for different single-cell applications. 3D reconstruction, dissociation of clusters, selective trapping/release, in situ analysis and pairing of single cells with barcode gel beads were demonstrated. To further verify the reliability and robustness of this technology in complex biosamples, the separation of circulating tumor cells from undiluted blood based on properties of both physics and immunity was achieved. With the rich selection of handling modes, the platform has the potential to be a full-process microsystem, from pretreatment to analysis, and used in numerous fields, such as in vitro diagnosis, high-throughput single-cell sequencing and drug development.”

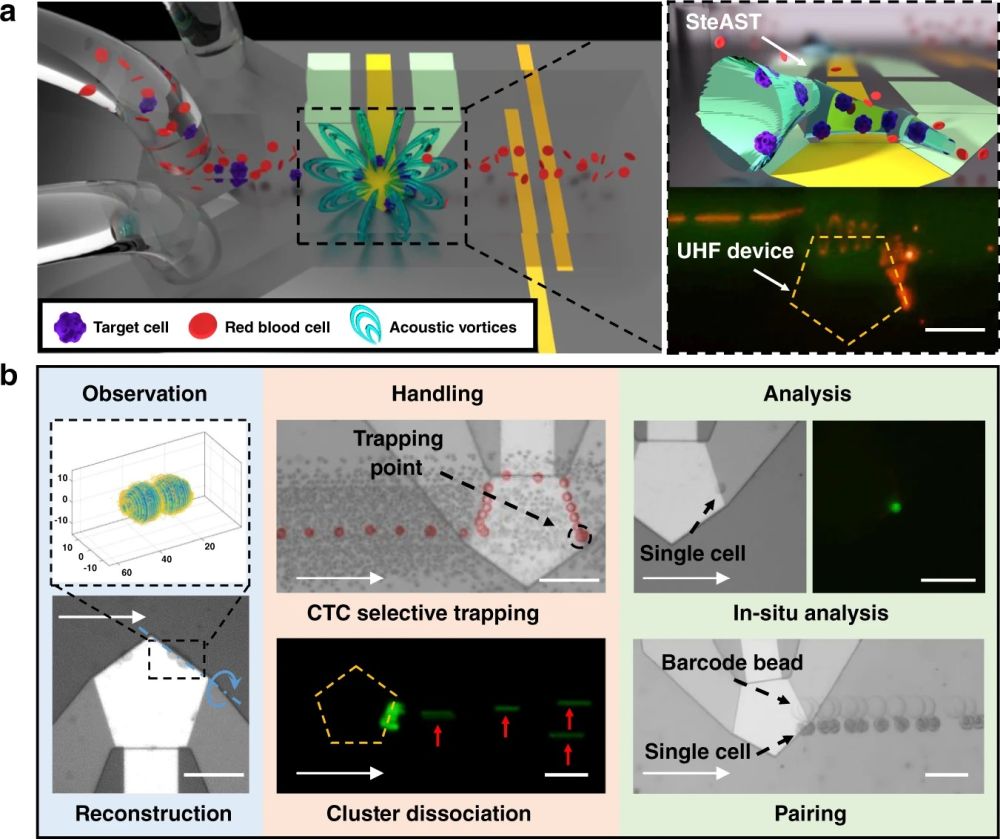
a A schematic of the SteAST platform. The SteAST platform comprises a microfluidic channel bonded to a UHF BAW resonator on a silicon substrate. The attenuation of BAWs in a coupled liquid triggers 3D acoustic streaming vortices. The detailed images show a cartoon profile of SteAST and the stacked image that demonstrates the trajectory of a fluorescent particle (5 μm) in the tunnel to describe the actual profile of SteAST. b SteAST-based multimode manipulation of single cells. The reconstruction of the cluster was achieved via rotation manipulation, demonstrating successful 3D observation. As a demonstration of the pretreatment of biological samples, this platform provides size-based selective trapping of a single cell and shear force-based dissociation of clusters. To show successful analysis, in situ analysis of single cells by staining and pairing a target cell with a barcode gel bead for downstream analysis was demonstrated with the cooperation of the customized microfluidic channel. White arrows show the direction of lateral flow. The blue dotted line and blue arc arrows show the axis and direction of rotation, respectively. The red arrows point to the individual cells after dissociation. The scale bar is 100 µm.” Reproduced under Creative Commons Attribution 4.0 International License from Yang, Y., Pang, W., Zhang, H. et al. Manipulation of single cells via a Stereo Acoustic Streaming Tunnel (SteAST). Microsyst Nanoeng 8, 88 (2022).
Figures and the abstract are reproduced from Yang, Y., Pang, W., Zhang, H. et al. Manipulation of single cells via a Stereo Acoustic Streaming Tunnel (SteAST). Microsyst Nanoeng 8, 88 (2022). https://doi.org/10.1038/s41378-022-00424-9
Read the original article: Manipulation of single cells via a Stereo Acoustic Streaming Tunnel (SteAST)


