09 Jun Microphysiological in vitro platform for studying sarcopenia
Abstract
“Microphysiological systems (MPS), also referred to as tissue chips, incorporating 3D skeletal myobundles are a novel approach for physiological and pharmacological studies to uncover new medical treatments for sarcopenia. We characterize a MPS in which engineered skeletal muscle myobundles derived from donor-specific satellite cells that model aged phenotypes are encapsulated in a perfused tissue chip platform containing platinum electrodes. Our myobundles were derived from CD56+ myogenic cells obtained via percutaneous biopsy of the vastus lateralis from adults phenotyped by age and physical activity. Following 17 days differentiation including 5 days of a 3 V, 2 Hz electrical stimulation regime, the myobundles exhibited fused myotube alignment and upregulation of myogenic, myofiber assembly, signaling and contractile genes as demonstrated by gene array profiling and localization of key components of the sarcomere. Our results demonstrate that myobundles derived from the young, active (YA) group showed high intensity immunofluorescent staining of α-actinin proteins and responded to electrical stimuli with a ~1 μm displacement magnitude compared with non-stimulated myobundles. Myobundles derived from older sedentary group (OS) did not display a synchronous contraction response. Hypertrophic potential is increased in YA-derived myobundles in response to stimulation as shown by upregulation of insulin growth factor (IGF-1), α-actinin (ACTN3, ACTA1) and fast twitch troponin protein (TNNI2) compared with OS-derived myobundles. Our MPS mimics disease states of muscle decline and thus provides an aged system and experimental platform to investigate electrical stimulation mimicking exercise regimes and may be adapted to long duration studies of compound efficacy and toxicity for therapeutic evaluation against sarcopenia.”
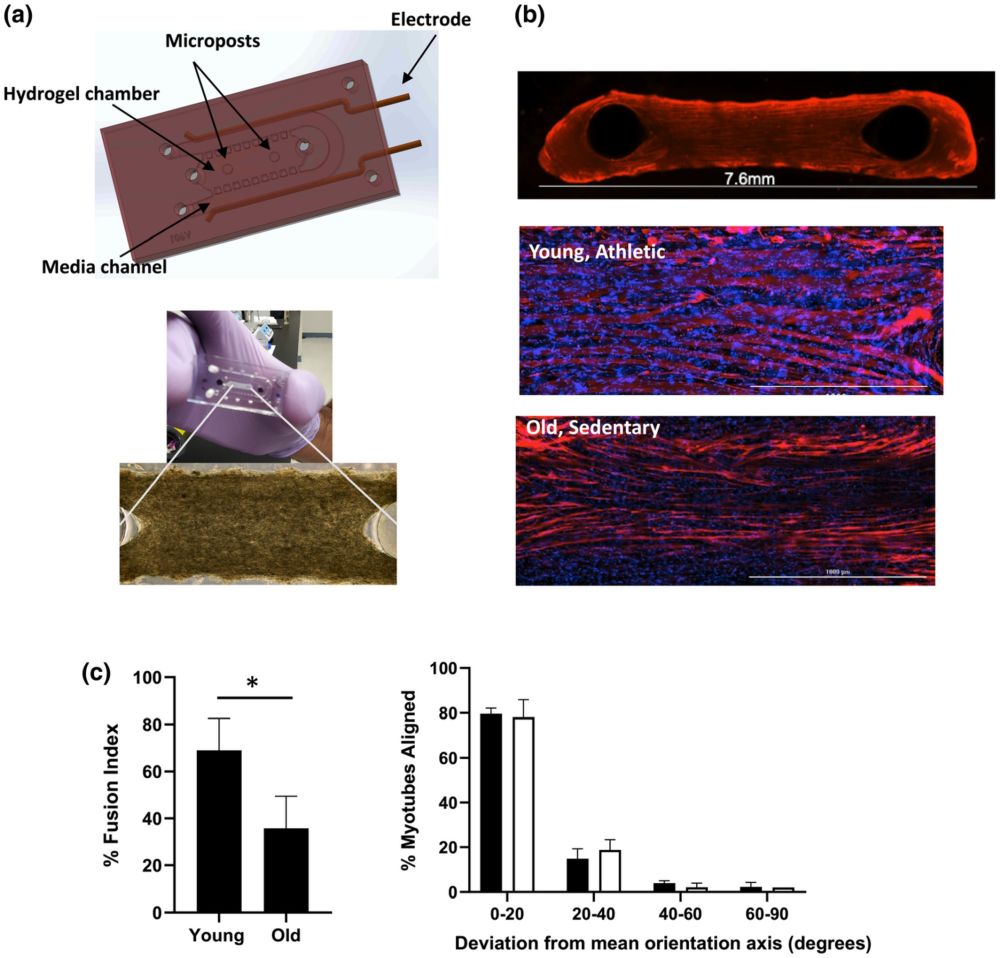
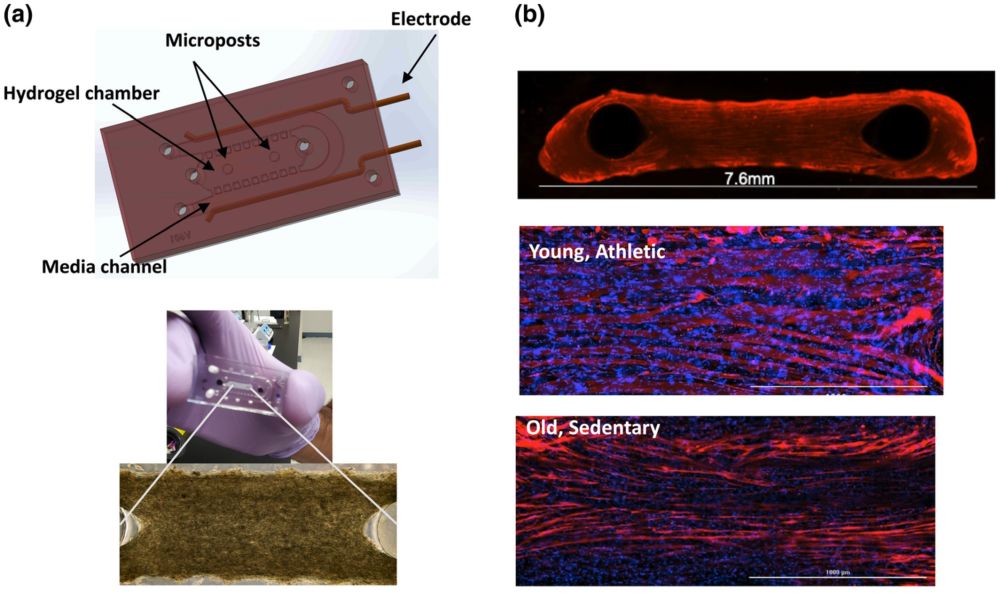
“Characterization of YA- and OS-derived muscle myobundles in microfluidic chip. a upper) Computer-assisted design rendering of microfluidic PDMS chip indicating where the myobundles are formed around the two posts and a lower) a representative phase contract image of myobundle formation after 2 days in culture. b top) Representative (4×) image of OS-derived myobundle after differentiation phase and immunofluorescence stained with MF-20 (red) and b middle) Immunostaining with MF-20 (red) and Dapi (blue) of YA-derived myobundle and b bottom) OS-derived myobundles (20×, Scale bar 1000 μm). c left) Percent fusion index determined by immunostaining shown in b and spot counting algorithm. Data are average ± standard deviation of seven chips per pooled cell cohort (*p < 0.05 analyzed by two-tailed paired t-test). c right) Mean alignment score showing the deviation of the longitudinal myotube axis from the mean orientation axis (line between posts) for YA-derived myobundles (black bars) and OS-derived myobundles (white bars). Values are calculated as a percent of total myotubes per sample. Data average of three individual chips per cohort.” Reproduced under Creative Commons Attribution 4.0 International License from , , , , , , , & (2022). Microphysiological system for studying contractile differences in young, active, and old, sedentary adult derived skeletal muscle cells. Aging Cell, 00, e13650.
Figures and the abstract are reproduced from Giza, S., Mojica-Santiago, J. A., Parafati, M., Malany, L. K., Platt, D., Schmidt, C. E., Coen, P. M., & Malany, S. (2022). Microphysiological system for studying contractile differences in young, active, and old, sedentary adult derived skeletal muscle cells. Aging Cell, 00, e13650. https://doi.org/10.1111/acel.13650 under Creative Commons Attribution 4.0 International License.
Read the original article: Microphysiological system for studying contractile differences in young, active, and old, sedentary adult derived skeletal muscle cells


