14 May A microfluidic platform to investigate effect of mechanobiological cues on the dynamics of angiogenesis
Mechanobiological cues can be of crucial importance after an injury for regulating wound angiogenesis. However, mechanoregulation of angiogenesis is not fully understood in wound healing. Microfluidics has shown to be the optimal choice when it comes to mimicking the cellular microenvironment and investigating the effect of mechanical, chemical, electrical, etc. on cellular activities. Here, in a recent publication in Nature Communications, a research group developed a microfluidic chip for investigating the effect of intraluminal pressure on wound angiogenesis. By mimicking the blood flow-driven intraluminal pressure (IP) in a microfluidic environment, the team could analyze the dynamics of angiogenesis upstream and downstream of the wound.
“Angiogenesis is regulated in coordinated fashion by chemical and mechanical cues acting on endothelial cells (ECs). However, the mechanobiological mechanisms of angiogenesis remain unknown. Herein, we demonstrate a crucial role of blood flow-driven intraluminal pressure (IP) in regulating wound angiogenesis. During wound angiogenesis, blood flow-driven IP loading inhibits elongation of injured blood vessels located at sites upstream from blood flow, while downstream injured vessels actively elongate.“, the authors explained.
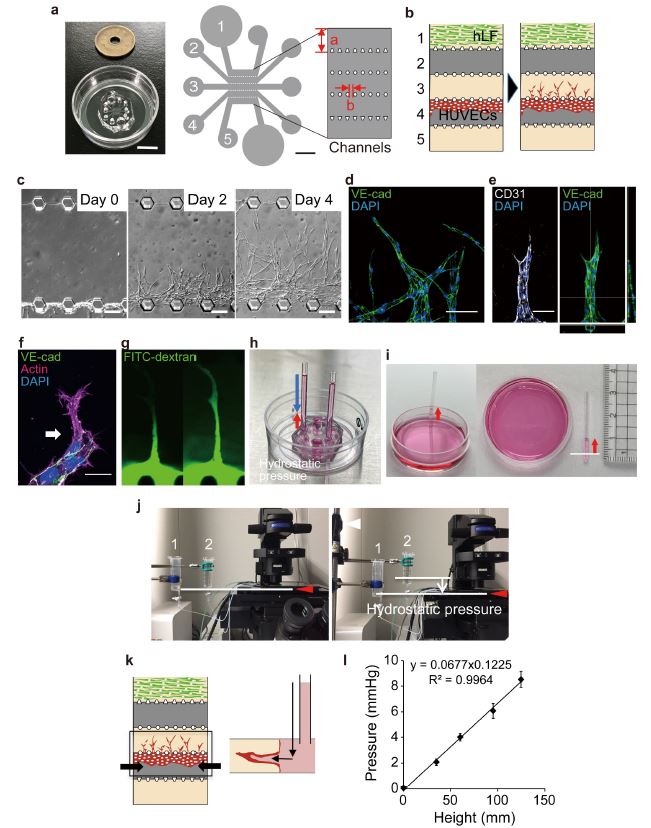
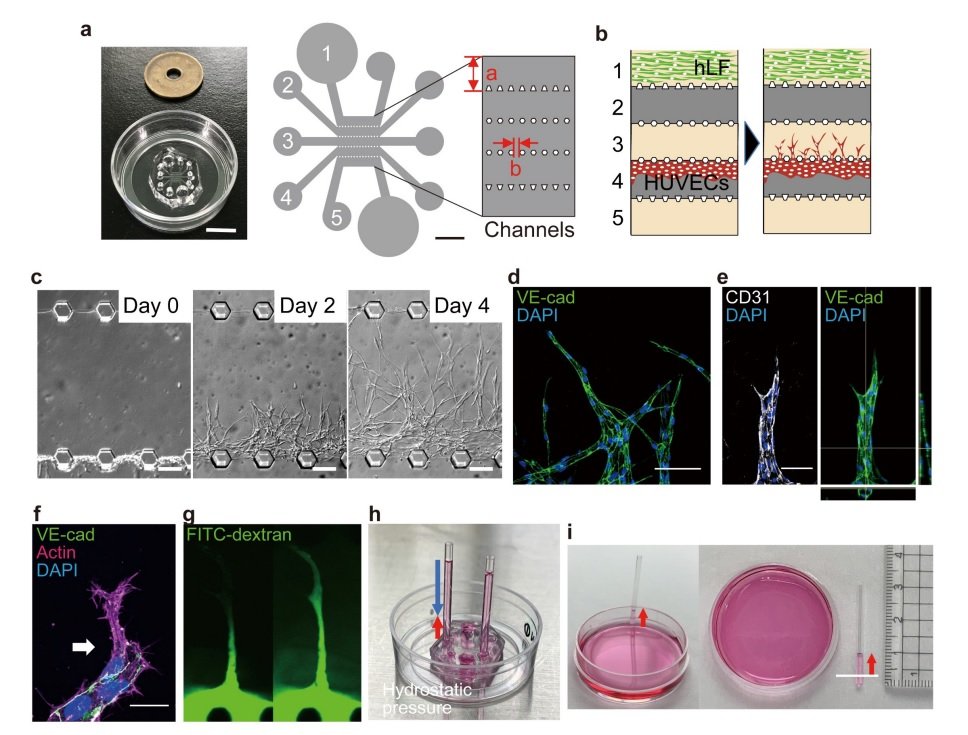
On-chip angiogenesis assay and vascular intraluminal pressure loading systems. a Microfluidic device mounted on a 35 mm glass bottom culture dish (left). Schematic illustration of top view of the device design (right). Five parallel channels (each 700 μm in width, a) were partitioned by microposts (100 μm window, b). b Schematic illustration of on-chip angiogenesis assay. In the on-chip angiogenesis assay, HUVECs seeded on Channel 4 migrate into the fibrin-collagen matrices filling Channel 3 and form angiogenic branches in response to soluble angiogenic factors secreted from human lung fibroblasts (hLF) in Channel 3. c Representative DIC images showing on-chip angiogenesis. Angiogenic branches which formed in Channel 3 before (Day 0) and 2 (Day 2) and 4 (Day 4) days after induction of angiogenesis are shown. d-f Confocal z-projection images and the orthogonal views (right in e) of angiogenic sprouts in an on-chip angiogenesis assay. d, the merged image of VE-cadherin (VE-cad, green) and DAPI (blue); e, the merged image of CD31 (white) and DAPI (blue) (left) and that of VE-cadherin (VE-cad, green) and DAPI (blue) (right); f, the merged image of VE-cadherin (VE-cad, green), actin (magenta), and DAPI (blue). Note that an angiogenic sprout has a lumen (e). An arrow in f indicates a tip EC. g Fluorescent images showing visualization of the vascular lumen by introducing FITC-dextran-containing angiogenic medium into channel 4. FITC-dextran diffused from the root of the angiogenic branch (left) and then toward the tip (right) for 1 sec through the lumen. h-l Devices for applying hydrostatic pressure to the lumen of elongating vessels. h, i Fixed type. Hydrostatic pressure (blue arrow in h) is applied to the lumens of angiogenic sprouts by placing capillaries filled with media (25 mm) in channel 4. Considering the negative pressure (red arrows in h and i, approximately 0.6 mmHg) generated by the capillary phenomenon (white line in i, water surface in culture dish), approximately 1.2 mmHg hydrostatic pressure is loaded on the lumens of angiogenic branches. j Variable type. Hydrostatic pressure was induced by height differences between the water surfaces in syringe 1 and syringe 2. White lines, water surfaces in syringes. k Schematic of hydrostatic pressure (arrows) loading system on microfluidic device (left, top view and right, side view). l Validation of the variable type of hydrostatic pressure loading system, using a differential pressure gauge. Data are presented as means ± s.e.m. (n = 5 devices examined over 5 independent experiments). Source data are provided as a Source Data file. Scale bars: 10 mm (left in a) and 200 μm (right in a), 100 μm (c, d), 20 μm (e, f). Reproduced from Choudhury, M.I., Li, Y., Mistriotis, P. et al. Kidney epithelial cells are active mechano-biological fluid pumps. Nat Commun 13, 2317 (2022). under Creative Commons Attribution 4.0 International License.
“In this study, we uncovered a novel molecular mechanism governing the mechanical regulation of EC behavior during wound angiogenesis. By exploiting a recently developed live imaging system for adult zebrafish20, we analyzed the dynamics of angiogenic EC behavior during wound healing and surprisingly discovered that elongation of severed blood vessels is preferentially induced downstream from blood flow, whereas blood flow-driven intraluminal pressure (IP) loading suppresses elongation of upstream injured vessels by inducing EC stretching.“, the authors explained.
Figures were reproduced Yuge, S., Nishiyama, K., Arima, Y. et al. Mechanical loading of intraluminal pressure mediates wound angiogenesis by regulating the TOCA family of F-BAR proteins. Nat Commun 13, 2594 (2022). https://doi.org/10.1038/s41467-022-30197-8 under Creative Commons Attribution 4.0 International License.
Read the original article: Mechanical loading of intraluminal pressure mediates wound angiogenesis by regulating the TOCA family of F-BAR proteins


