07 May A microfluidic platform reveals: Active mechanobiological fluid pumping via kidney cells
The interplay between mechanical forces that result in kidney epithelial fluid transport is not fully understood. In this week’s research highlight, we will introduce a recent microfluidic advancement published in Nature Communications that investigates how renal epithelial cells can serve actively as cellular pumps and generate hydraulic pressure gradients across the epithelium.
“we developed a microfluidic platform to study the mechanics of fluid transport by epithelial cells. The device is designed to simultaneously measure fluid flow across the epithelium and careful control/monitoring of hydraulic pressures at the apical and basal sides of the epithelium. Using this device, we show that kidney epithelial cells are active mechanobiological fluid pumps, i.e., they generate a hydraulic pressure gradient across the epithelium (opposite of the fluid flow direction), and the pump stalls when the pressure gradient is too high.“, the authors explained.
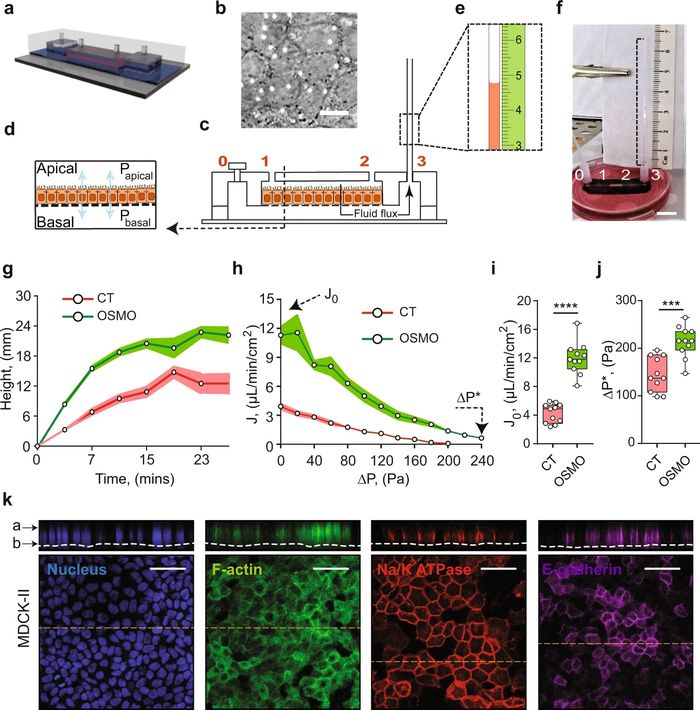
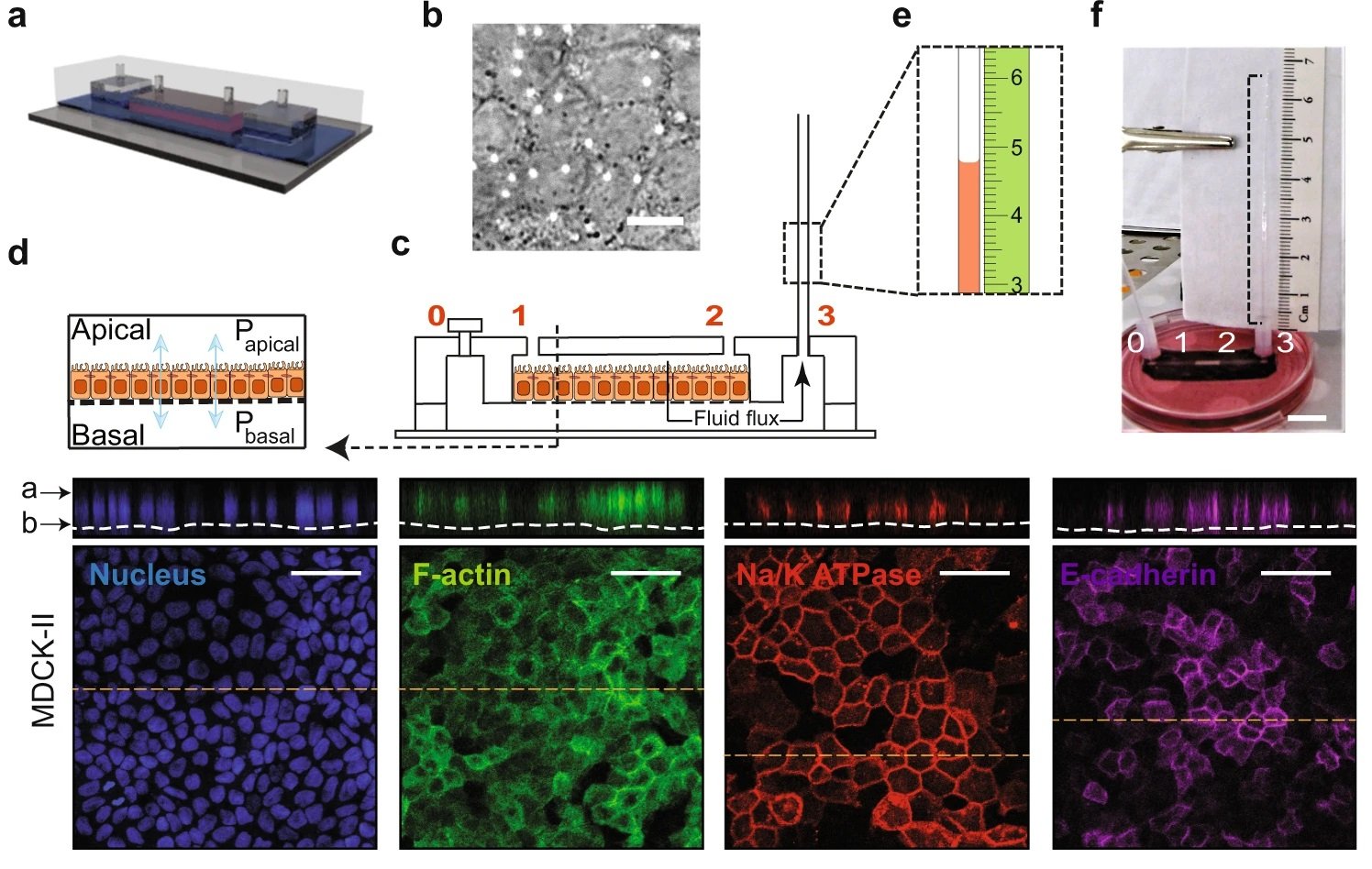
a A schematic representation of the Micro-fluidic Kidney Pump (MFKP). b A DIC image of the MDCK-II monolayer. White dots indicate pores. Scale bar = 10 mm. c Longitudinal section of the device. Black arrows indicate fluid flux. d A schematic of a polarized epithelium. e Dashed rectangle shows zoomed schematic of fluid flow in the microcapillary (MC) and a mm scale (green). f A snapshot of the MFKP inside an incubator for time-lapsed videography. Scale bar = 1 cm. g The height of fluid in the MC is plotted as function of time for MDCK-II epithelium in MFKP. Compared to static condition (CT, red) (n = 11, N = 3, devices, biological replicates), the fluid pumping action changed when the apical medium is switched to 20% hypo-osmotic condition (OSMO, green) (n = 11, N = 3). Shaded area is the standard error of the mean (SEM). h Pump performance curve (PPC) of MDCK-II epithelium, showing the measured trans-epithelial fluid flux (J) (apical to basal) as a function of the hydrostatic pressure gradient (ΔP = Pbasal − Papical). J0 is the fluid flux at zero pressure gradient (ΔP = 0) and ΔP* is the stall pressure when J = 0. Both J0 and ΔP* change in the hypo-osmotic condition. Shaded area is the SEM. Comparison of J0 (i) and ΔP* (j) for MDCK-II epithelium in the static condition (CT, red) (n = 11, N = 3, devices, biological replicates,) and 20% apical hypo-osmotic condition (OSMO, green) (n = 11, N = 3, devices, biological replicates, p = 0.0002). ****p < 0.0001 and ***p < 0.001 (two-tailed Mann–Whitney t-test). k Immunofluorescence (IF) images showing nuclei (blue), F-actin (green), NKA (red) and E-cadherin (purple) in MDCK-II epithelium in the MFKP. Scale bar = 25 mm. For the box plots, lower and upper box boundaries 25th and 75th percentiles, respectively, line inside box median, lower and upper error lines 10th and 90th percentiles, filled circles indicate data points, respectively. Reproduced from Choudhury, M.I., Li, Y., Mistriotis, P. et al. Kidney epithelial cells are active mechano-biological fluid pumps. Nat Commun 13, 2317 (2022). under Creative Commons Attribution 4.0 International License.
The working mechanism of the microfluidic device
The team aimed at developing a microfluidic device that emulates a relevant microphysiological environment for nephron tubular epithelia in the kidney to measure the fluid flux and hydrostatic pressure across the epithelium. The proposed microfluidic chip consists of four parts. The top and bottom microfluidic chambers were microfabricated with PDMS using conventional soft lithography. These two microfluidic chambers were separated by a porous membrane in between. The whole microfluidic assembly was then bonded to a glass slide. The top microfluidic chamber was pre-coated with fibronectin to facilitate cell growth and adhesion. Next, the cells were seeded in the top microfluidic channel and were given time to reach confluency. The bottom microfluidic channel was connected to a microcapillary through the outlet port to measure the pressure.
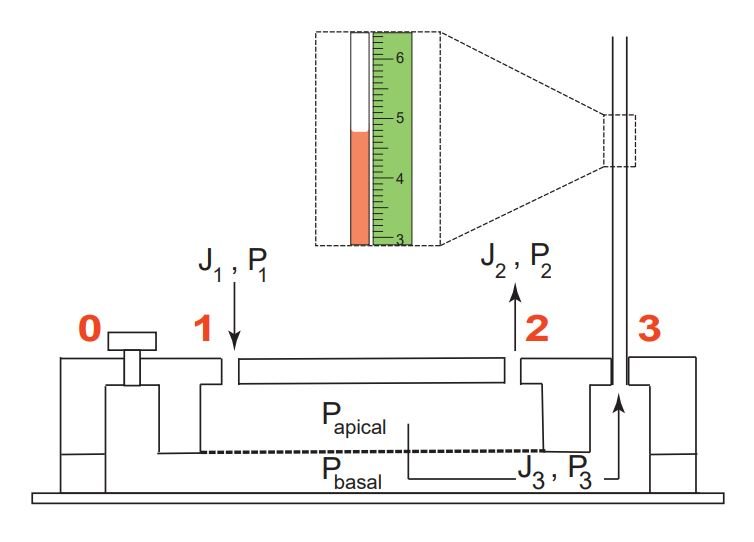
Dashed box indicates a zoomed section of the fluid flow in the MC and a mm scale. J1 and P1 are the fluid flux and hydrostatic pressure at port 1, J2 and P2 are the fluid flux and hydrostatic pressure at port 2, J3 and P3 are the fluid flux and hydrostatic pressure at port 3. Reproduced from Choudhury, M.I., Li, Y., Mistriotis, P. et al. Kidney epithelial cells are active mechano-biological fluid pumps. Nat Commun 13, 2317 (2022). under Creative Commons Attribution 4.0 International License.
First, the inlet port of the bottom microfluidic channel was sealed after filling the channels with the culture media. Next, the top microfluidic channel was connected to a syringe pump and a range of flow rates and shear stresses were applied to calibrate the pressure read-out on the microcapillary. Due to the hydrostatic pressure, the flow in the top microfluidic channel will cause the media to pass through the membrane and penetrate the bottom microfluidic channel. This would in turn increase the pressure in the bottom microfluidic channel and will cause the height of the fluid column to rise inside the microcapillary.
“Our results demonstrate that secretory and absorptive functions of epithelia can generate significant mechanical forces, and these forces may be important in tubular morphogenesis in general. Our combined results offer insights into the mechanics of kidney epithelial fluidic pumping action, hydraulic pressure gradient transduction and the mechanobiology of ADPKD cyst development. “, the authors explained.
Figures were reproduced from Choudhury, M.I., Li, Y., Mistriotis, P. et al. Kidney epithelial cells are active mechano-biological fluid pumps. Nat Commun 13, 2317 (2022). https://doi.org/10.1038/s41467-022-29988-w under Creative Commons Attribution 4.0 International License.
Read the original article: Kidney epithelial cells are active mechano-biological fluid pumps


