11 Apr Leveraging microfluidics to analyze potential materials for capturing microplastics
Abstract
“Microplastics accumulate in various aquatic organisms causing serious health issues, and have raised concerns about human health by entering our food chain. The recovery techniques for the most challenging colloidal fraction are limited, even for analytical purposes. Here we show how a hygroscopic nanocellulose network acts as an ideal capturing material even for the tiniest nanoplastic particles. We reveal that the entrapment of particles from aqueous environment is primarily a result of the network’s hygroscopic nature – a feature which is further intensified with the high surface area of nanocellulose. We broaden the understanding of the mechanism for particle capture by investigating the influence of pH and ionic strength on the adsorption behaviour. We determine the nanoplastic binding mechanisms using surface sensitive methods, and interpret the results with the random sequential adsorption (RSA) model. These findings hold potential for the explicit quantification of the colloidal nano- and microplastics from different aqueous environments, and eventually, provide solutions to collect them directly on-site where they are produced.”
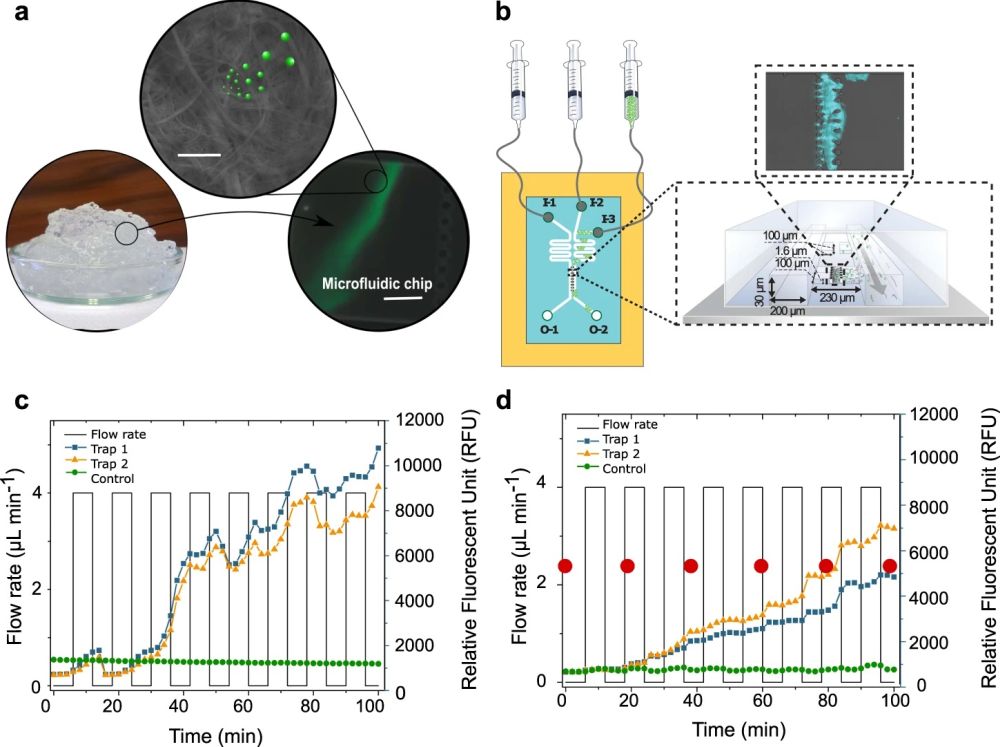
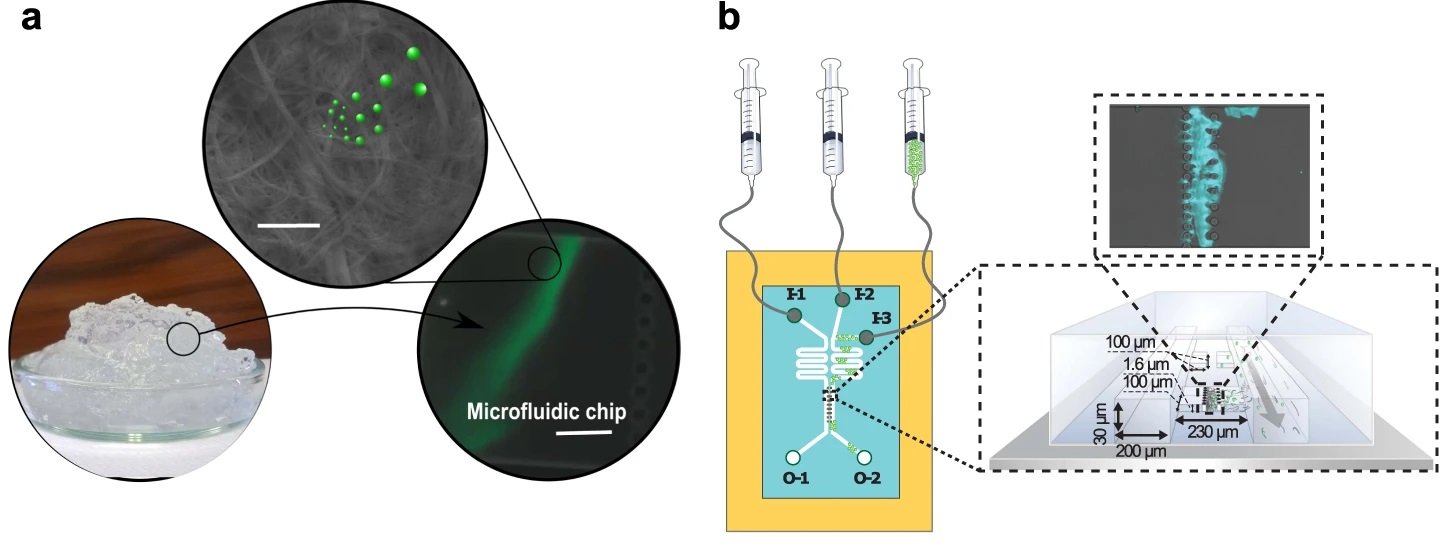
a Schematic illustration of a proof of concept where the capture of fluorescently labeled polystyrene (PS) nano- and microplastic particles (PS(ø100 nm) and PS(ø1µm)) by CNF hydrogel network is verified using a microfluidic set-up and fluorescent imaging (Supplementary Video 1). Scale bar in the scanning electron microscope (SEM) image is 1 µm and, 25 µm in the microfluidic chip image. b Schematic illustration of the microfluidic setup for CNF containing trap showing the injection of fluorescent PS particles (I-3) and water (I-2/I-1). I-1 channel is used to pack the CNF hydrogel into the connected traps and I-2 is used for washing. Fluorescent accumulation of cationic PS(ø100 nm) (c) and PS(ø1µm) (d) over time by CNF hydrogel network. Green curves show control trap without CNF hydrogel. The orange and blue curves show parallel experiments with CNF in the traps. In (d), the red dots indicate the time points where microscopy images were taken (Supplementary Fig. 1). C.J. created the syringes in Fig. 1b using the ChemDraw software, version 20.1.1. from PerkinElmer Informatics. Source data are provided as a Source Data file. Reproduced under Creative Commons Attribution 4.0 International License from Leppänen, I., Lappalainen, T., Lohtander, T. et al. Capturing colloidal nano- and microplastics with plant-based nanocellulose networks. Nat Commun 13, 1814 (2022).
Figures and the abstract are reproduced from Leppänen, I., Lappalainen, T., Lohtander, T. et al. Capturing colloidal nano- and microplastics with plant-based nanocellulose networks. Nat Commun 13, 1814 (2022). https://doi.org/10.1038/s41467-022-29446-7 under Creative Commons Attribution 4.0 International License.
Read the original article: Capturing colloidal nano- and microplastics with plant-based nanocellulose networks


