31 Mar Integrating Acoustic Manipulation with Microfluidics for Single-Cell Analysis
One of the most promising applications of microfluidics technology has been proven to be the single-cell analysis. Hardly a week goes by without a new microfluidic development for microfluidic single-cell analysis. Over the nearly past decade, the field has accelerated at a high rate and continued to facilitate single-cell analysis for genomics, transcriptomics, proteomics, etc. for researchers. A recent publication in Nature Communications reports a novel advancement for microfluidic single-cell analysis.
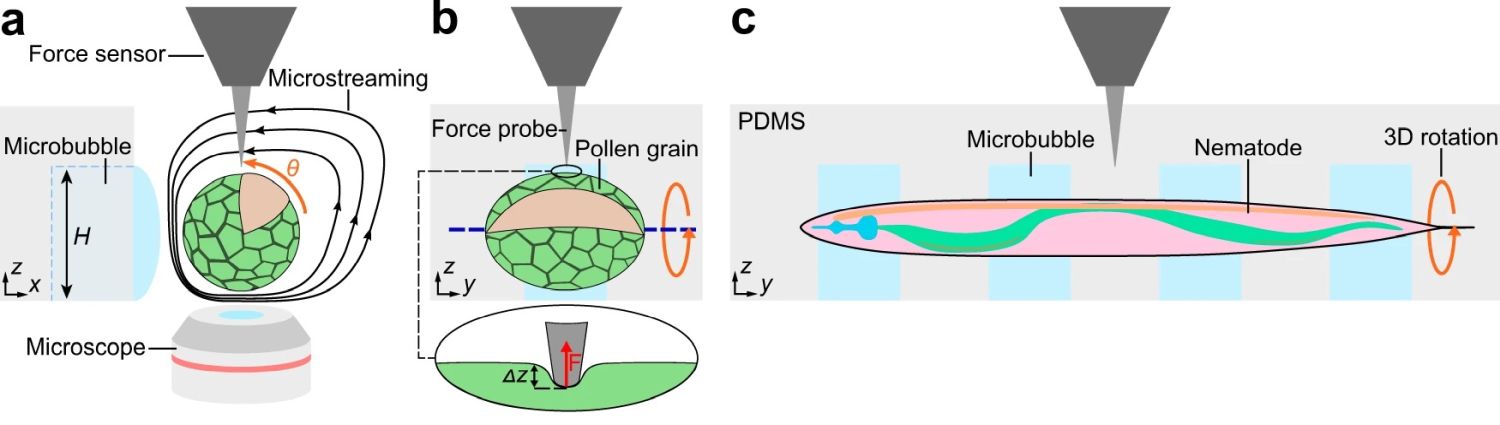
A Zurich-based research group takes advantage of the combination of a microfluidic chip with open microchannels and acoustic manipulation for 3D mechanical characterization of single-cells and small organisms. The study focuses on using acoustic manipulation, a non-invasive method, to handle single cells and small organisms like pollen grains and nematodes. This method is seamlessly integrated with microfluidic devices, enabling precise control and manipulation within a microfluidic chip environment.
“Here, we combine an acoustically driven manipulation device with a micro-force sensor to freely rotate biological samples and quantify mechanical properties at multiple regions of interest within a specimen. The versatility of this tool is demonstrated through the analysis of single Lilium longiflorum pollen grains, in combination with numerical simulations, and individual Caenorhabditis elegans nematodes.”, the authors explained.
Reproduced under Creative Commons Attribution 4.0 International License. Läubli et al., Nat. Commun., 2021.
The open microfluidic device was made from PDMS using conventional microfluidic microfabrication methods. The open microchannel contained a linear array of rectangular microcavities which were used to trap microbubbles. The hydrophobic/hydrophilic interaction caused the locally confined microbubbles to stay trapped inside the microcavities during the experiment. The specimen was then introduced to the microchannel and positioned near the microbubbles. A piezo transducer placed near the microfluidic device excited the microbubbles resulting in the formation of microvortices near the microbubble and in the surrounding liquid as shown in the image. These microvortices exerted force on the specimen and resulted in rotation of the specimen.
Alongside acoustic manipulation, the researchers employed force microscopy. This combination allows for a detailed analysis of the mechanical properties of biological samples, exploring aspects like cell elasticity and organismal responses to mechanical stress. This innovative approach opens new avenues in understanding the biomechanics of living systems. It exemplifies how microfabrication and microfluidic technology can be harnessed for sophisticated biological analyses. The integration of acoustic manipulation with force microscopy in a microfluidic setup represents a leap forward in cell analysis and biomechanical research, offering potential applications in medical diagnostics, drug testing, and more.
“ Our results highlight the importance of manipulation capabilities during mechanical characterization to prevent misinterpretations or simplifications in modeling. Additionally, the applications could be expanded to the investigation of spheroids of transformed cells, with a view to quantifying mechanical alterations in cancer cells. Moreover, improvements in the imaging system will provide additional views (e.g., side views) during indentation that will dramatically simplify subsequent analyses.”, the authors explained.
Read the original article: 3D mechanical characterization of single cells and small organisms using acoustic manipulation and force microscopy


