26 Mar A microenigneered system models multicellular events in early pregnancy
The complex process of human pregnancy begins with the crucial step of embryo implantation. This involves the intricate interaction between the embryo’s trophoblast cells and the maternal endometrium. A groundbreaking study published in Nature Communications has developed an advanced microfluidic chip, termed “implantation-on-a-chip”, to simulate and study this vital process, offering unprecedented insights into early pregnancy. This study marks a significant advancement in reproductive biology by developing a microphysiological model that closely replicates the early stages of human pregnancy. The implantation-on-a-chip is a microengineered system designed to mimic the three-dimensional structural organization of the maternal-fetal interface.
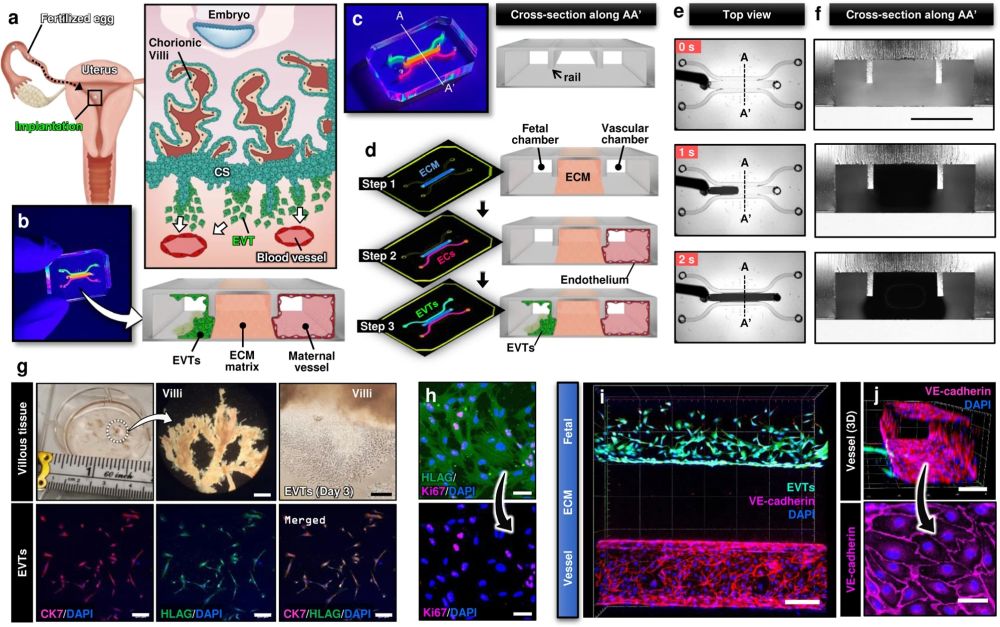
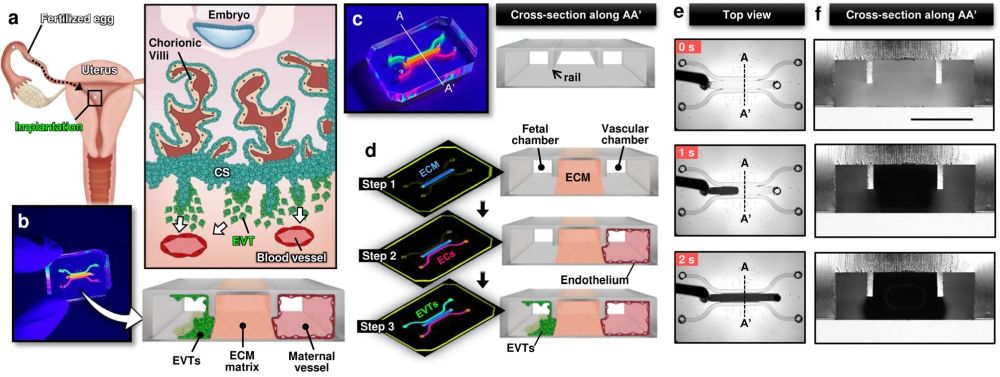
a Soon after implantation, EVTs begin to differentiate from precursor cells in the cytotrophoblast shell (CS) and invade into the uterus, a process that continues through the first half of pregnancy. b Compartmentalized design of the implantation-on-a-chip device for in vitro modeling of EVTs and a maternal SA separated by maternal endometrium. c Architecture of the implantation-on-a-chip microdevice. The center and two side lanes have dimensions of 0.5 mm (width) × 0.3 mm (height) and 0.25 mm (width) × 0.3 mm (height), respectively. d Sequential steps of model construction. e Time-lapse imaging of ECM hydrogel precursor (colored black) injection into the center lane of the device. f Images of device cross-section to show capillary pinning-based physical confinement of injected hydrogel solution (dark solution) in the center lane. Scale bar, 500 μm g (Top row) Photos of first trimester termination tissue and EVT outgrowth from the tissue explants. Scale bars, 1 mm (middle) and 200 μm (right). (Bottom row) The purity of the population was confirmed by immunostaining for cytokeratin 7, a trophoblast marker (magenta) and HLA-G, an EVT-specific marker (green). The representative images of the villous tissue are from five independent experiments. Scale bars, 200 μm. h Immunostaining of HLA-G (green) and Ki67 (magenta) expression by EVTs cultured on coverslips in a 6 well plate, after 3 passages. The representative images of EVTs are from four independent experiments. Scale bars, 50 μm. i Top-down confocal projection of the microengineered maternal-fetal interface at Day 1. EVTs in the fetal chamber were labeled with CellTracker Green (green). ECs were stained for VE-cadherin (magenta). The representative image is from three independent devices. Scale bar, 200 μm. j Endothelial tube in the vascular compartment at Day 1. Magenta and blue show VE-cadherin and nuclear staining, respectively. The representative images are from three independent devices. Scale bars, 100 μm (top) and 50 μm (bottom). EVTs: Extravillous trophoblasts, CS: Cytotrophoblastic shell, ECs: Endothelial cells, ECM: Extracellular matrix, CK7: Cytokerain 7, HLA-G: human leukocyte antigen G.. Reproduced under Creative Commons Attribution 4.0 International License from Park, J.Y., Mani, S., Clair, G. et al. A microphysiological model of human trophoblast invasion during implantation. Nat Commun 13, 1252 (2022).
The microfluidic device was intricately designed using cutting-edge microfluidic microfabrication techniques. The microfluidic device was microfabricated using PDMS and consisted of multiple layers of biocompatible materials, precisely constructed to create a network of microchannels and chambers. These microstructures are engineered to house and sustain primary human cells, replicating the environment of the maternal uterus and the invading fetal extravillous trophoblasts. The researchers utilized primary human cells isolated from clinical specimens, ensuring a high degree of physiological relevance. The device allowed for the controlled interaction of these cells, simulating the complex process of trophoblast invasion into the maternal endometrium. By adjusting various parameters within the microenvironment, the team was able to study how different conditions affect trophoblast migration and behavior.
The implantation-on-a-chip features microengineered maternal vessels to observe the invasion of extravillous trophoblasts. Its design enables the study of cellular interactions necessary for vascular remodeling during implantation. The device allows for real-time observation and analysis of cell behavior, providing insights that were previously unattainable with conventional in vitro models. The study demonstrated the in vivo-like directional migration of extravillous trophoblasts, a critical aspect of successful implantation. It revealed the role of decidualized stromal cells as regulators of trophoblast migration. The research also brought to light the influence of maternal immune cells on the process of trophoblast invasion. These findings have significant implications for understanding and potentially treating pregnancy complications.
The implantation-on-a-chip microfluidic model represents an advancement in the study of human reproduction. By providing a detailed simulation of the maternal-fetal interface using advanced microfluidic techniques, this microfluidic system opens new doors for research into the early stages of pregnancy, enhancing our understanding of reproductive health and disease.
Figures and the abstract are reproduced from Park, J.Y., Mani, S., Clair, G. et al. A microphysiological model of human trophoblast invasion during implantation. Nat Commun 13, 1252 (2022). https://doi.org/10.1038/s41467-022-28663-4 under Creative Commons Attribution 4.0 International License.
Read the original article: A microphysiological model of human trophoblast invasion during implantation


