08 Jan Toxicological evaluation of chemicals and cosmetics on a microfluidic skin-on-a-chip
Organ-on-a-chip microfluidic devices are well known for their abilities in mimicking in vivo conditions and their superiority in drug toxicity screening compared to regular cell culture models. In this week’s research highlight, we will introduce another organ-on-chip that takes advantage of microfluidics technology to mimic the complex and multilayered human skin for the toxicological evaluation of chemical drugs and cosmetics.
“Animal models are prohibited worldwide for testing cosmetics. Therefore, reliable human skin models that recapitulate physiological events in skin tissue need to be established under in vitro settings. In this study, hybrid human skin models that enable delicate toxicological evaluations of drugs and cosmetic compounds are demonstrated. To recapitulate skin cornification, keratinocytes in the top layer of a vertical microfluidic chip were cultured at the air–liquid interface. “, the authors explained.

Reproduced with permission from J. S. Lee, J. Kim, B. Cui, S. K. Kim, S. Cho, S. An and S. Cho, Lab Chip, 2022, Advance Article , DOI: 10.1039/D1LC00550B
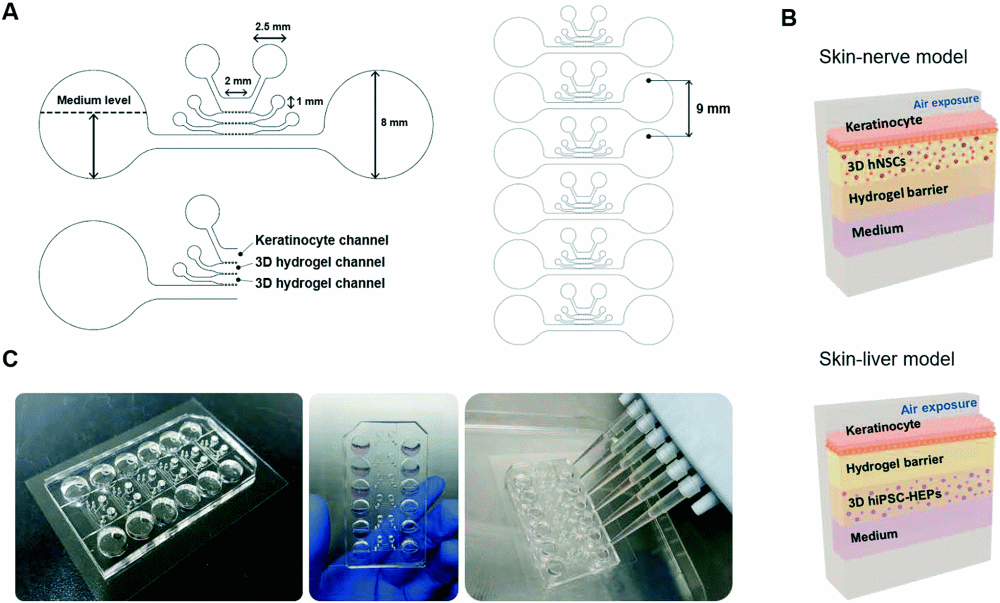
The working mechanism of the microfluidic device
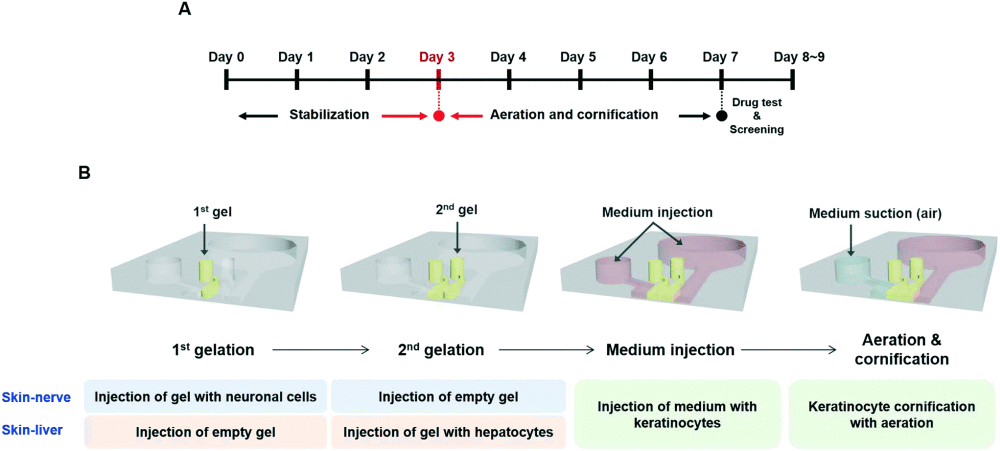
The aim of this study was to develop hybrid skin models mimicking the in vivo physiology for toxicological evaluations. The forms a complex matrix consisting of multiple layers. Therefore, as shown above, the proposed microfluidic device consisted of multiple microfluidic compartments for culturing different cell types. Two different versions of the microfluidic chip were used in the study; one to model the skin-nerve interactions and the other one to investigate the interplay between the skin and liver in the presence of the drugs. In both cases, the same microfluidic chip was used with different cell types. The skin mimicking microfluidic device consisted of four parallel microchannels made with PDMS connected with micron-sized barriers. In both the skin-nerve and skin-liver scenarios, the topmost and the bottommost microchannels were used as the skin layer and for introducing the medium, respectively. In the skin layer, keratinocytes were cultured and exposed to aeration to induce cornification. In the skin-nerve microfluidic model, the second from the top channel was filled with human Neural stem cells (hNSCs) within a collagen matrix and the third channel was filled with an empty hydrogel matrix. However, in the skin-liver case, the second microchannel housed the empty hydrogel matrix while human-induced pluripotent stem cell-derived hepatocyte-like cells (hiPSC-HEPs) within a hydrogel matrix were introduced to the third microchannel. Each microfluidic chip comprises 6 of these microfluidic devices to allow simultaneous analysis.
Reproduced with permission from J. S. Lee, J. Kim, B. Cui, S. K. Kim, S. Cho, S. An and S. Cho, Lab Chip, 2022, Advance Article , DOI: 10.1039/D1LC00550B
First, in both of the microfluidic skin models, several markers were screened to prove functionality on the chip. Neuronal cells expressed several biomarkers such as Tuj1 and MAP2 as well as electrophysiological response to the neurotransmitter glutamate. Likewise, hiPSC-HEPs expressed mature hepatic markers (HN4A, albumin) confirming the maturity and functionality of the cells on the chip. Moreover, in order to mimic the top layer of the skin, first, the keratinocytes were stabilized in the medium for three days. This was followed by removal of the medium and exposure to air to induce certification and form a thick epidermis layer.
The ability of the microfluidic skin chip was shown by evaluating the drug toxicity response to tretinoin. Tretinoin is a chemical used for exfoliating dead skin cells and is generally administered in a dose of 0.1%. The skin-nerve microfluidic chip was exposed to 0.1%, 1%, and 10% doses and the overall structure was monitored. It was shown that at 0.1%, the structure was maintained while at higher doses, the skin layer collapsed indicating complete exfoliation exhibiting a similar response to native skin. The microfluidic chip was then evaluated for skin sensitization and hepatotoxicity and was shown in both cases to deliver promising results. All in all, the microfluidic skin chip was shown to be provide a useful model for human skin with potential applications in the pharmaceutical and cosmetic industries.
Read the original article: Hybrid skin chips for toxicological evaluation of chemical drugs and cosmetic compounds


