13 Nov Microfluidic device mimics pitting of malaria parasites in the spleen
Malaria can introduce inclusion bodies in red blood cells which can be repaired in the spleen in a process called pitting. In the pitting process, a particle can get removed from the cytoplasm of the cells without destroying the cell itself. In this week’s research highlight, we will introduce a recent article published in the Scientific Reports journal where a research team reports a simple and user-friendly microfluidic chip that can be used to mimic the pitting of malaria parasites in red blood cells that occurs in the spleen.
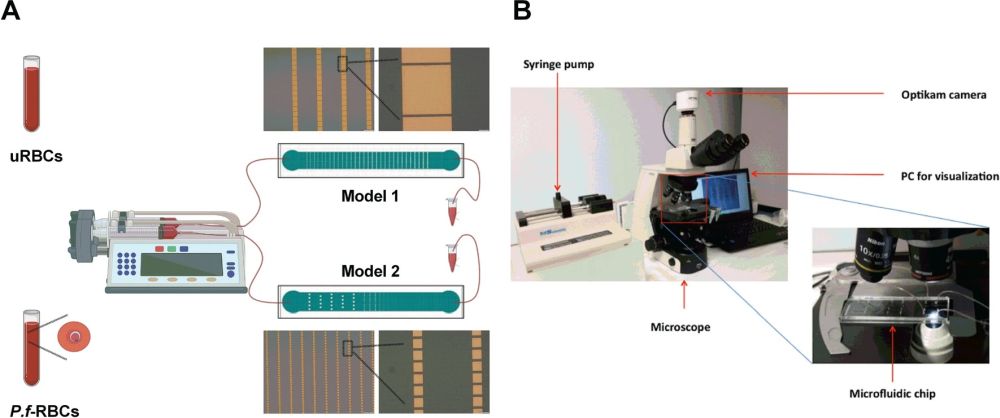
” To study this physiological function in vitro, we have developed two microfluidic devices modeling the IES, according to the hypothesis that at a certain range of mechanical stress on the RBC, regulated through both slit size and blood flow, would force it undergo the pitting process without affecting the cell integrity. To prove its functionality in replicating pitting of malaria parasites, we have performed a characterization of P. falciparum-infected RBCs (P.f.-RBCs) after their passage through the devices, determining hemolysis and the proportion of once-infected RBCs (O-iRBCs), defined by the presence of a parasite antigen and absence of DAPI staining of parasite DNA using a flow cytometry-based approach. “, the authors explained.
Reproduced from Elizalde-Torrent, A., Trejo-Soto, C., Méndez-Mora, L. et al. Sci Rep 11, 22099 (2021). under Creative Commons Attribution 4.0 International License.
The working mechanism of the microfluidic device
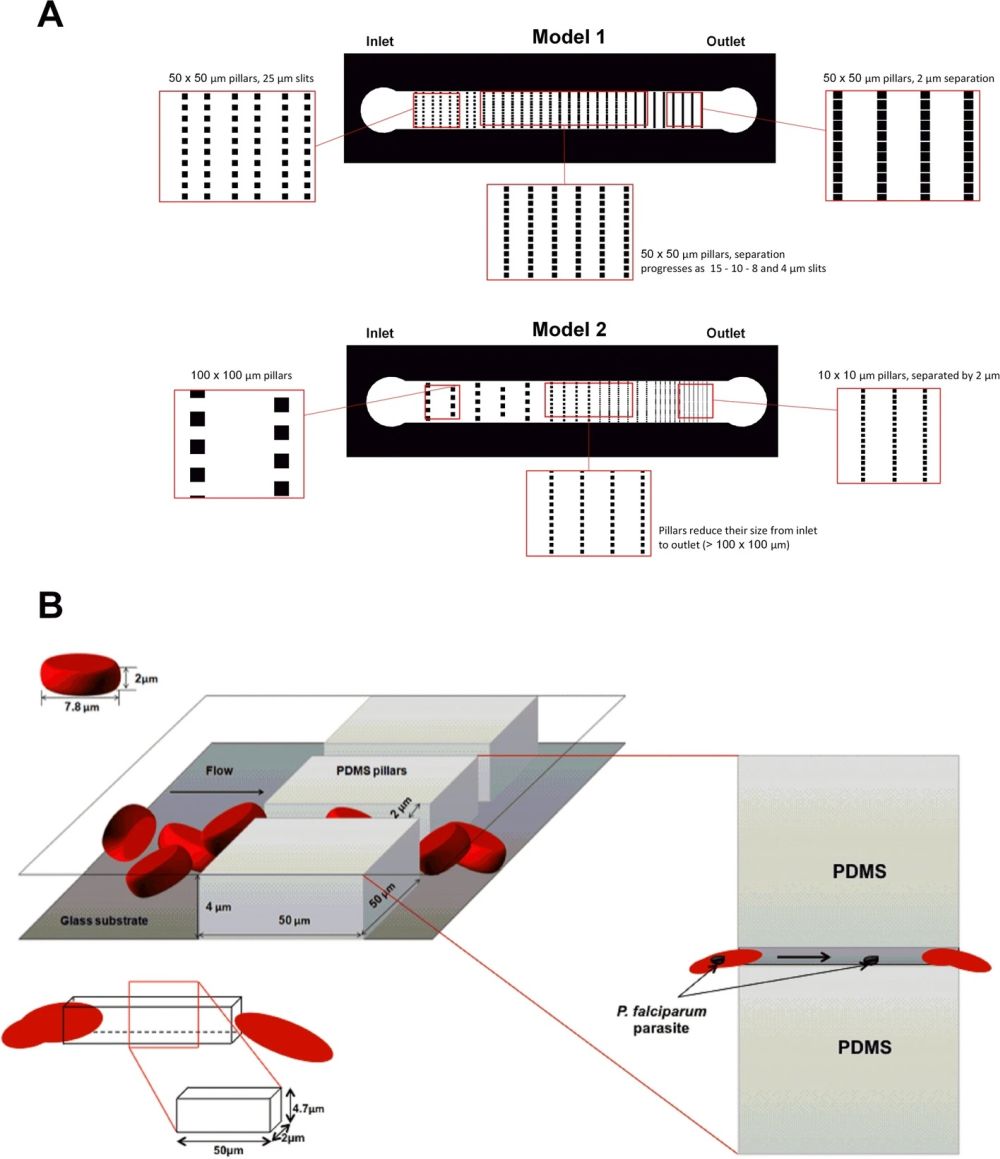
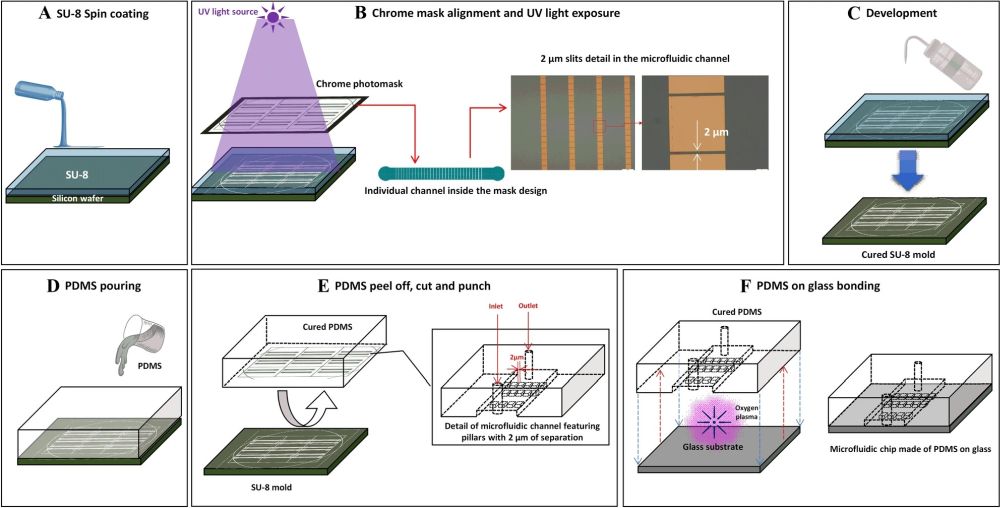
Two PDMS microfluidic devices were microfabricated for this research. Conventional photolithography and soft-lithography techniques were used for the microfabrication of the microfluidic chips. The first microfluidic chip consisted of a microchannel with a width of 1 mm and a length of 8mm. A set of micropillars were placed in the microchannel that formed the so-called slits. The micropillars had a fixed dimension throughout the microchannel (50 µm×50 µm). However, the slit size varied from 50 µm at the beginning to 2 µm at the end. The second microfluidic chip had the same total dimension as the first one. However, the size of the micropillars varied along the length of the microchannel. The micropillars’ dimensions varied from 100 µm×100 µm to 10 µm×10 µm. The slit size decreased accordingly from 100 µm to 2 µm. In the first design, the large and constant size of the micropillars results in an increase in the times required for the cells to pass through the slits. Therefore, the cells experience a longer exposure to deformability stress. On the other hand, in the second microfluidic chip, the micropillar size decreases while the width of the device is constant. This leads to an increase in the number of slits and an increase in the frequency of pitting. The height of the microchannels was selected such that the cells do not have the chance to rotate inside the device.
Reproduced from Elizalde-Torrent, A., Trejo-Soto, C., Méndez-Mora, L. et al. Sci Rep 11, 22099 (2021). under Creative Commons Attribution 4.0 International License.
The microfluidic chips were then tested with various conditions. Blood was run through the device at 5 and 200 µL/min. Hemolysis analysis of the RBCs after being collected from the device indicated that they remain intact after passing through the microfluidic device. The cells were analyzed using flow cytometry to determine whether pitting occurred in the microfluidic devices. The results suggest that passing the P. falciparum-infected RBCs through the microfluidic device resulted in the loss of parasites.
“Although pitting is a classic concept in the literature, little is known about the details of this process itself. Thanks to this work, we have now a better understanding of this process, both from the physical and biological point of view. Furthermore, we have been able to replicate the pitting of P.f.- RBCs thanks to the portable microfluidic devices, which prompts us to think that our design thus has applications for exploring pathophysiology in malaria and other hemolytic anemias where pitting corrects for red blood cells with solid particles in the cytoplasm without destructing the cell.“, the authors concluded.
Figures are reproduced from Elizalde-Torrent, A., Trejo-Soto, C., Méndez-Mora, L. et al. Pitting of malaria parasites in microfluidic devices mimicking spleen interendothelial slits. Sci Rep 11, 22099 (2021) under Creative Commons Attribution 4.0 International License
Read the original article: Pitting of malaria parasites in microfluidic devices mimicking spleen interendothelial slits


