08 Sep Droplet microfluidic enables high-throughput generation of mammary tumor organoids in microbeads
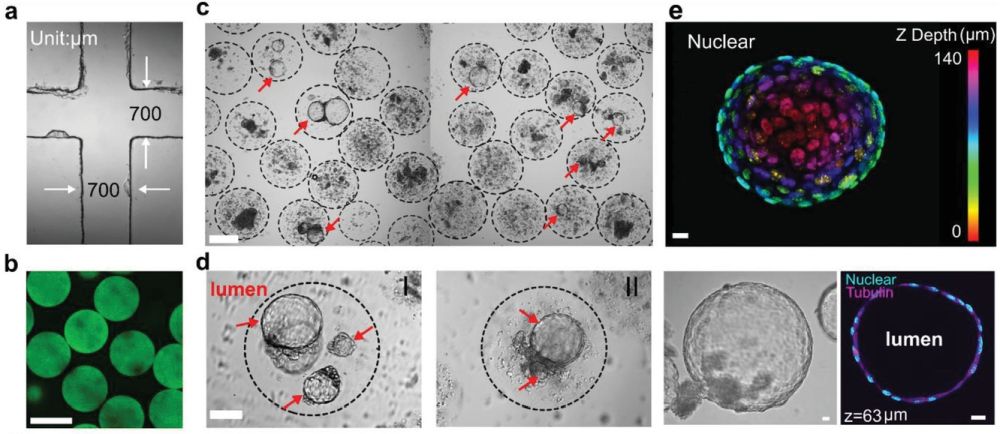
“Mammary tumor organoids have become a promising in vitro model for drug screening and personalized medicine. However, the dependency on the basement membrane extract (BME) as the growth matrices limits their comprehensive application. In this work, mouse mammary tumor organoids are established by encapsulating tumor pieces in non-adhesive alginate. High-throughput generation of organoids in alginate microbeads is achieved utilizing microfluidic droplet technology. Tumor pieces within the alginate microbeads developed both luminal- and solid-like structures and displayed a high similarity to the original fresh tumor in cellular phenotypes and lineages. The mechanical forces of the luminal organoids in the alginate capsules are analyzed with the theory of the thick-wall pressure vessel (TWPV) model. The luminal pressure of the organoids increase with the lumen growth and can reach 2 kPa after two weeks’ culture. Finally, the mammary tumor organoids are treated with doxorubicin and latrunculin A to evaluate their application as a drug screening platform. It is found that the drug response is related to the luminal size and pressures of organoids. This high-throughput culture for mammary tumor organoids may present a promising tool for preclinical drug target validation and personalized medicine.
”
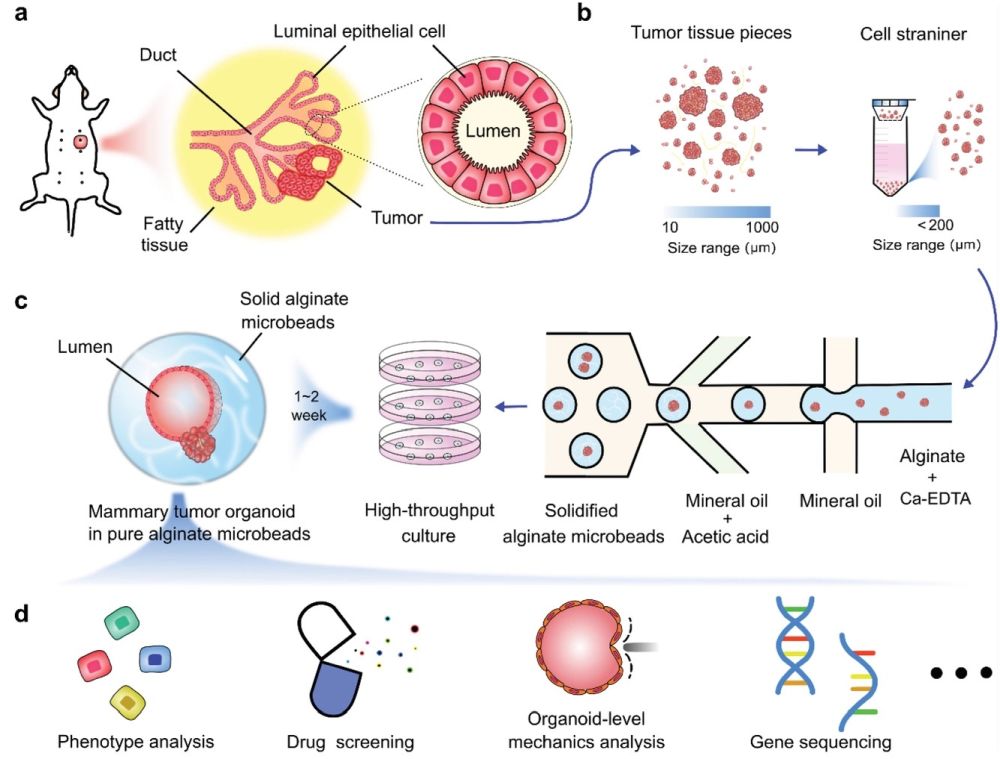
“Schematic of high throughput culture of mammary tumor organoids in alginate microbeads. a) Schematic for the structure of mouse mammary tumor. b) Tumor tissues were chopped into pieces and passed through cell strainers. c) High-throughput generation of alginate microbeads with tumor pieces encapsulated by the microfluidic droplet technique. After 1–2 weeks of culture, mammary tumor organoids formed luminal structures in the alginate microbeads. d) Potential application of the mammary tumor organoids in alginate microbeads. ” Reproduced under Creative Commons Attribution 4.0 International License. Fang et al., Adv. Sci., 2021.
Figures and the abstract are reproduced from Fang et al., Adv. Sci., 2021 under Creative Commons Attribution 4.0 International License.
Read the original article: Mammary Tumor Organoid Culture in Non-Adhesive Alginate for Luminal Mechanics and High-Throughput Drug Screening


