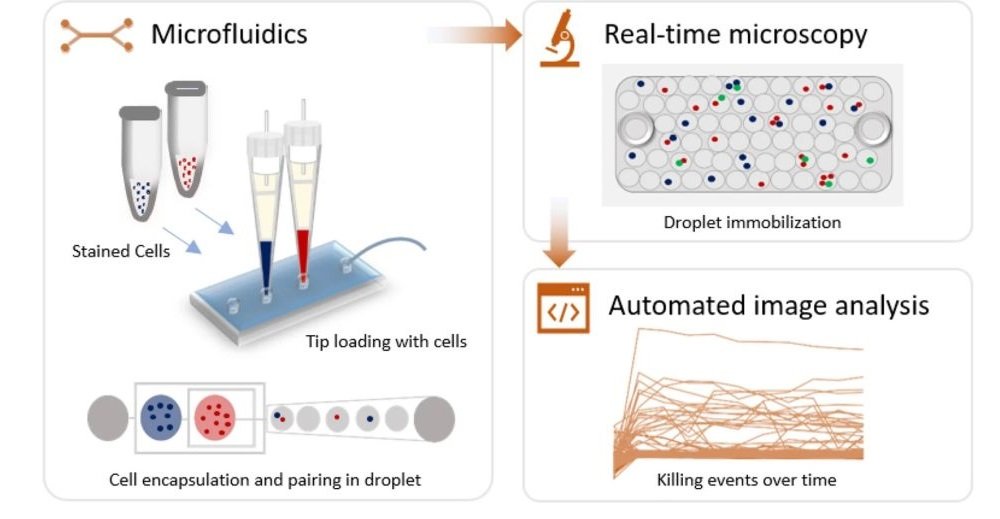
25 Aug Microfluidics enables real-time analysis of single natural killer cells
“Cytotoxicity is a vital effector mechanism used by immune cells to combat pathogens and cancer cells. While conventional cytotoxicity assays rely on averaged end-point measures, crucial insights on the dynamics and heterogeneity of effector and target cell interactions cannot be extracted, emphasizing the need for dynamic single-cell analysis. Here, we present a fully automated droplet-based microfluidics platform that allowed the real-time monitoring of effector-target cell interactions and killing, allowing the screening of over 60,000 droplets identifying 2000 individual cellular interactions monitored over 10 h. During the course of incubation, we observed that the dynamics of cytotoxicity within the Natural Killer (NK) cell population varies significantly over the time. Around 20% of the total NK cells in droplets showed positive cytotoxicity against paired K562 cells, most of which was exhibited within first 4 h of cellular interaction. Using our single cell analysis platform, we demonstrated that the population of NK cells is composed of individual cells with different strength in their effector functions, a behavior masked in conventional studies. Moreover, the versatility of our platform will allow the dynamic and resolved study of interactions between immune cell types and the finding and characterization of functional sub-populations, opening novel ways towards both fundamental and translational research.”
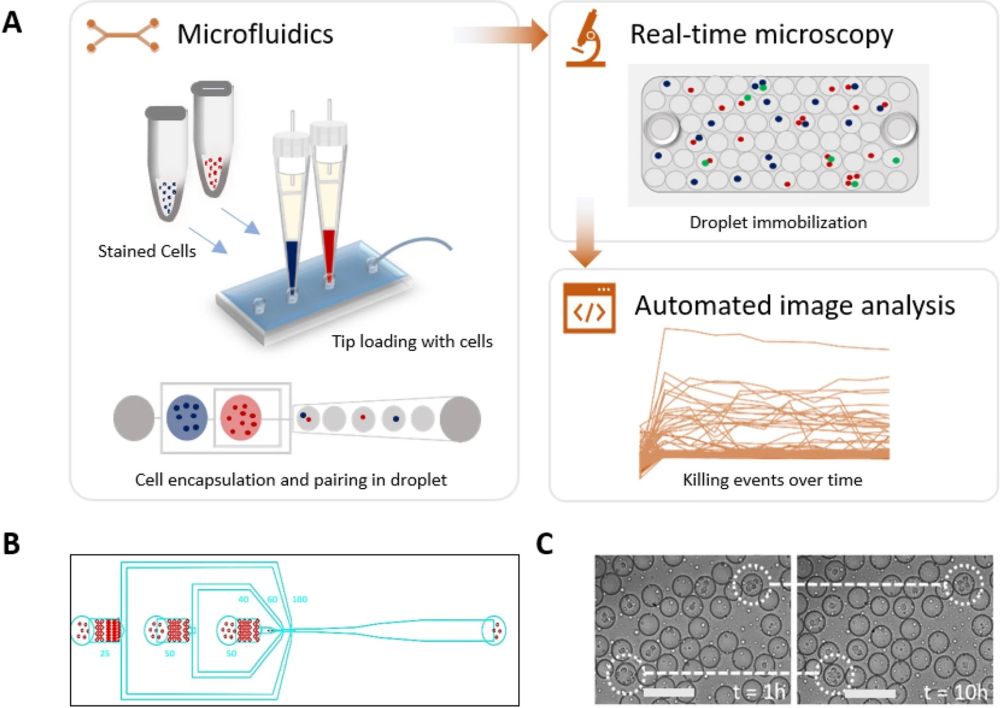
“Experimental setup of high-throughput droplet-based cytotoxicity platform. (A) Experimental schematics showing cytotoxicity platform that combines (i) droplet generation and cell pairing using microfluidics, (ii) droplet immobilization for real-time microscopy, (iii) automated image analysis using custom-made MATLAB script to allow unbiased and high throughput detection of cytotoxic events. Stained NK cells and K562 cells were loaded into the chip using 200 µL pipette tips and encapsulated into droplets using a 3-inlet microfluidic chip. The viability dyes were included within the cell medium. The immobilized droplets were incubated in a stage top incubator set at 5% CO2 and 37 °C. Image acquisition was performed at every hour interval for 10 h. (B) The three-inlet microfluidic device with flow-focusing junction to generate droplets. (C) A qualitative test of the observation chamber was performed by monitoring droplets movement in the chamber under the microscope for 10 h.” Reproduced under Creative Commons Attribution 4.0 International License. Subedi et al., Sci. Rep., 2021.
Read the original article: An automated real-time microfluidic platform to probe single NK cell heterogeneity and cytotoxicity on-chip


