Limits of 3D printing in prototyping and manufacturing microfluidics
The area of biological and chemical analysis is undergoing miniaturization in a fashion similar to integrated circuits in electronics. In this regard Microfluidics offers the most important contributions in addition to saving reagent consumption, reducing waste generation, process integration, and reducing costs [1]. Conventional methods for fabrication of microfluidic devices include casting of PDMS/silicone, injection molding or hot embossing of thermoplastics (eg PMMA, polycarbonate, or COC/COP), wet or dry etching of glass or silicon, and micromachining or laser engraving. Although these methods are very effective and have been optimized to fit numerous applications, large scale manufacturing of Microfluidics can cause bottlenecks and slow development of microfluidics products. For over 30 years, the large community of Microfluidics has been in search for newer and more effective manufacturing processes that offer reduced cost per chip in conjunction with micron-scale spatial resolution. The highly anticipated game changing fabrication method should allow for cheaper, easier, and faster iterative prototyping and manufacturing of microfluidic devices in different materials [2]. Three-dimensional (3D) printing, aka additive manufacturing, is a layer-by-layer fabrication process developed in the early 1980s that has over the past 5–10 years emerged as a promising approach for the fabrication of microfluidic devices. Compared to conventional manufacturing methods, it is attractive for the fabrication of micro mechanical systems because of ability to fabricate more complex and bespoke designs, including those with integrated functionality, and the ability to create truly 3D structures in a matter of minutes or hours[3].
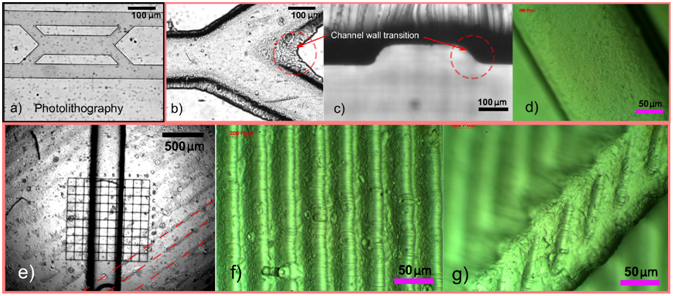
a) Photolithography, and b-f) stereo-lithographic (SLA) 3D printing. Images b-d) show channels manufactured using SLA molds printed in-plane with the substrate to achieve smooth surfaces. Images e-g) show characteristic ridges of channels manufactured using SLA molds printed at an angle to the substrate plane to improve print resolution. Credit to Plos One: https://doi.org/10.1371/journal.pone.0245206
There are two obvious merits for 3d printing. First is the capability of fabricating in three dimensions in a way that has not been previously possible. This presents new opportunities in the field of microfluidics as researchers begin to imagine what might be possible when manipulating surfaces and fluids in three dimensions. The second feature – the ability to rapidly realize a model – enables researchers to adopt a “fail fast and often” strategy. In this method a simple fluidic device can be printed in 10-15 min, while more complex ones may take hours. The printing may be done in a material that is more similar to thermoplastics than PDMS suggesting translation into commercial outcomes may be simpler. The most widely used 3D printing techniques in microfluidics include stereolithography (SLA), fused deposition modeling (FDM), inkjet printing, laminated object manufacturing (LOM), selective laser sintering (SLS), and direct writing. Each 3D printing technique has its own merits and drawbacks in terms of fabrication speed, resolution, accuracy, and cost.[3] Despite of all these benefits, 3D printed structures cannot currently compete with the resolution of structures produced by conventional lithography in a build space that is practical. In this article we review some concerns regarding dimensional accuracy, shape conformity, surface quality, biocompatibility, optical transparency and material availability[4] As an example Figure demonstrate microfluidics that were printed using SLA compared to a typical microfluidic chip made using photolithography.
Are there challenges for producing microfluidic devices using 3D printing technology?
3D printing can help to produce microfluidic chips in two ways: 1) Help with fabrication of molds, 2) Mold-less fabrication of microfluidic devices via direct printing. The most notable advantage of 3D printing is obviously the quick turnaround from concept to prototype. The 3D technology needs to overcome several challenges in order to be a game-changer in microfluidics production. Here we summarize those challenges.
How does print resolution affect the design and function of 3D printed microfluidic channels?
Although the resolution of many 3D-printers has been claimed to be as low as a few tens of microns, most of currently reported 3D-printed microfluidic devices have channel sizes from hundreds of microns to a few millimeters[6]. Achieving accurate dimensioning is an intrinsic challenge in 3D printing itself which becomes a hurdle to bring down the tolerance levels and to fabricate intricate 3D models at micro scale[7]. This is partially because the printers with the highest resolution usually need supporting materials to fill the void spaces (i.e., a channel) during the printing process, which typically needs to be removed manually. Unfortunately, it is almost impossible to remove all the waxy supporting materials from a very small channel. Given the challenges in removing waxy filler, any left-over material in a channel may adversely alter the flow pattern and/or affect the flowing reagents by absorption or reaction, and even contribute to channel blockage. Most of the supporting materials have similar chemical composition with the construction materials, which makes it impossible to use harsh solvents for cleaning. This limitation brings about several restrictions in designing and fabricating these microfluidic devices. For instance cell/particle gating and electrophoresis may be force to exclude sharp bends or Y-splits. If a channel with such structures as twists and turns must be fabricated, then it is best to print a feature layer (ie an engraved slab) and seal it with a flat substrate.[8]
Predictive modeling and printer specific process capability analysis can be a good starting point to reach the highest accuracy with repeatable prints and to minimize the deviations between the CAD design and printed structures. Moreover, novel methodologies like sub voxel gray-scale control can be used to manipulate the printer settings, specifically in DLP printers for 3D microfluidic channel printing.[9]
How is surface quality of microchannels fabricated by 3D printing?
Aside from the limitations around the resolution of 3D printing methods, one of the main issues with 3D-printing is the roughness of the surfaces [5]. Gross reported a microfluidic device fabricated using a high resolution (30 um on X and Y axes and 16 um on Z axis as said by the manufacturer) 3D-printer [13]. As shown in Figure, after removal of the supporting material, large rough ridges can be seen around the inner wall, which may cause issues such as dead volumes and inconsistent surface modifications. Reagent adsorption are another concern about surface properties of 3D-printed devices. An intrinsic feature of 3D printing devices made of acrylate and acrylonitrile butadiene styrene (ABS) is that they cause absorbing proteins and lipids and thereby altering detected signals or function of the assay. To avoid the bio toxicity concern of 3D-printed devices and to reduce channel roughness, some coating protocols can be applied. For example, Gross successfully coated a layer of PDMS on the inside of a 3D-printed channelfor endothelial cell culture and subsequent microscopicobservation.[8]
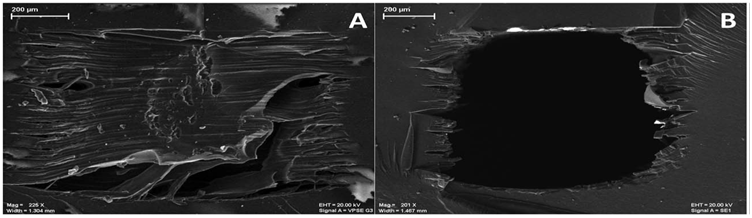
The SEM images of a 3D-printed channel before and after removing supporting material. Credit to Anal Chem: https://pubs.acs.org/doi/full/10.1021/acs.analchem.6b04344
Are there material compatibility issues using 3D printed Microfluidic chips?
Researchers are thriving towards making new materials for microfluidics keeping in mind properties such as biocompatibility for live cell studies. Still, a generic research on available materials for microfluidics for microfluidics manufacturing show a gap that needs more research to fill [10]. 3D-printing materials are usually multi-component mixtures. Even though the major composition is nontoxic, other components such as commonly used photo initiators may adversely affect cell viability. More biocompatible 3D-printing materials are being developed that are expected to be non-toxic to cells. However, most of these products are still in development. Solvent compatibility is another concern in 3D-printing. Some 3D-printing materials may swell in contact with liquid, which can cause flow problems [11]. Organic solvents are also commonly used in microfluidics, thus solvent compatibility of 3D-printing materials still remains a concern.[8]
How 3D printing is used to fabricate microfluidic chips indirectly?
Since closed channel geometries for microfluidics are difficult to fabricate reliably via 3D printing, researchers prefer to build only the molds via 3D printing instead of direct manufacturing of the 3D-Microfluidic devices. Alternatively, the channels can be printed on the surface of a layer sealed by a substrate in a fashion similar to conventional methods of fabrication of microfluidics. To do so, printers need to be of higher caliber. Such 3D printers are costlier, have lower throughput (to ensure the high resolution), and require routine maintenance and calibration. Higher accuracy (50 – 200 μm) closed-source Inkjet and FDM printers come in at least three times the price as compared to open-source desktop DLP or FDM printers (200 – 1000 μm accuracy). If the above-mentioned trade-off is made economically, direct fabrication of 3D structures becomes more feasible. [9]
And Finally, if there is no facility and no expertise in your institute to fabricate your own microfluidic devices, then it would be difficult to justify the costs unless you are establishing a new active microfluidic intensive program.
How easy is to use 3D printing technology by non-expert user?
3D printers come along with various controllable parameters, which need to be optimized for use. Some of them are material selection, temperature settings, software settings, STL resolution settings etc. This brings in the challenge of parameter optimization with respect to 3D-Microfluidic printing. In addition different technology or material system to produce the microfluidic device enforces certain maximum or minimum working temperatures for the chips that is not obvious to non experts.[9] As a result biologist or biomedical engineers who own a 3D printer in their laboratory need to closely collaborate with expert mechanical or plastics engineers to be able to utilize their system for microfluidics manufacturing.[9]
What are other issues with 3D printing of Microfluidics?
Cast PDMS or thermoplastic microfluidic devices can be completely transparent for optical detection. Although “clear materials” are available for 3D-printing, they are typically translucent/semi-transparent when printed. Labor-intensive polishing can make the outside of a device transparent and smooth; but the inner wall of a small, fully-sealed channel cannot be polished. In other words, it is almost impossible to optically image the inside of a 3d printed microfluidic channel without certain treatments. Finally, most of the 3D-printing materials are not gas permeable, which makes them not suitable for longer-term cell culture inside a channel without additional gas exchange supporting units.[8]
Conclusion: Could 3D printing be used for the production of Microfluidics?
Although 3D printing has been celebrated to revolutionize microfluidics by faster and cheaper prototyping of devices and offering pathways to bulk manufacturing, many improvements need to be made to the technology to be suitable for either low volume prototyping of Microfluidic chips in the common range of 10um-100um feature size, or for large volume production (+10,000) of devices with even larger features in the range of +200um. Currently the technology is not a candidate for large volume manufacturing of microfluidic devices and disposable cartridges due to very low throughput caused by printing speed and need for post process cleaning.
References
- S. Fiorini and . D. T. Chiu, “Disposable microfluidic devices: fabrication, function, and application,” BIOTECHNIQUES, vol. 38, no. 3, pp. 429-446, 2018.
- V. Nielsen, M. J. Beauchamp, G. P. Nordin and A. T. Woolley, “3D Printed Microfluidics,” Annual Review of Analytical Chemistry, vol. 13, pp. 45-65, 2020.
- Li, N. P. Macdonald, R. M. Guijt and M. C. Breadmore, “Increasing the functionalities of 3D printed microchemical devices by single material, multimaterial, and print-pause-print 3D printing,” Lab on a Chip, vol. 19, no. 35, pp. 35-49, 2019.
- Waheed, J. M. Cabot, N. P. Macdonald, T. Lewis, R. M.Guijt, B. Paull and M. C. Breadmore, “3D printed microfluidic devices: Enablers and Barriers,” Lab on a Chip, vol. 16, no. 11, pp. 1993-2013, 2016.
- Felton, . R. Hughes and . A. Diaz-Gaxiola, “Negligible-cost microfluidic device fabrication using 3D-printed interconnecting channel scaffolds,” PLOS ONE, vol. 16, no. 2, pp. 1-22, 2021.
- K Au, W. Huynh, . L. F Horowitz and A. Folch, “3D-Printed Microfluidics,” Angew Chem Int, vol. 55, no. 12, pp. 3862-81, 2016.
- Shahrain M, Didier T, Lim G and A. J. Qureshi , “FAST DEVIATION SIMULATION FOR ‘FUSED DEPOSITION MODELING’ PROCESS,” Procedia CIRP, 43, pp. 327-332, 2016.
- Chen, B. T. Mehl, A. S. Munshi, A. D. Townsend, D. M. Spenceb and R. S. Martin, “3D-printed microfluidic devices: fabrication, advantages and limitations—a mini review,” Analytical Methods, vol. 8, no. 31, pp. 6005-6012, 2016.
- S. Rupal, E. . A. Garcia, C. Ayranci and A. . J. Qureshi, “3D Printed 3D-Microfluidics: Recent Developments and Design Challenges,” Journal of Integrated Design and Process Science, vol. 22, no. 1, pp. 5-20, 2018.
- Kalsoom, P. N. Nesterenkoab and B. Paul, “Recent developments in 3D printable composite materials,” RSC Advances, vol. 6, no. 65, pp. 60355-60371, 2016.
- Migneault, A. Koubaa, F. Erchiqui, A. Chaala, K. Englund and M. P. Wolcott, “Effects of processing method and fiber size on the structure and properties of wood-plastic composites,” Composites Part A: Applied Science and Manufacturing, vol. 40, no. 1, pp. 80-85, 2009.
- S. Fiorini and D. T. Chiu, “Disposable microfluidic devices: fabrication, function, and application,” BioTechniques, vol. 38, no. 3, pp. 429-446, 2005.
- Gross BC, Anderson KB, Meisel JE, McNitt MI, Spence DM. Anal Chem. 2015;87:6335–6341.
PDMS has been the material of choice for Microfluidics Fabrication. Learn why?
PDMS is a hydrophobic, optically transparent elastomer. How it can be modified to be different?