24 Apr Multi-layer microfluidic chip to quantify protein secretion in circulating tumor cells (CTCs)
Personalized medicine aims at providing individual patients with a therapy tailored to their features. It can significantly increase the body’s response to the medicine and the therapeutic treatments and is even more pronounced in patients with cancer. The two arms of the personalized medicine are 1) a versatile pool of therapeutic approaches to select from and 2) understanding the features of each patient. For the latter, various diagnostic tools help in better understanding of the patient such as analyzing the circulating tumor cells (CTCs), circulating free DNA, and extracellular vesicles secreted from the cancerous tissue.
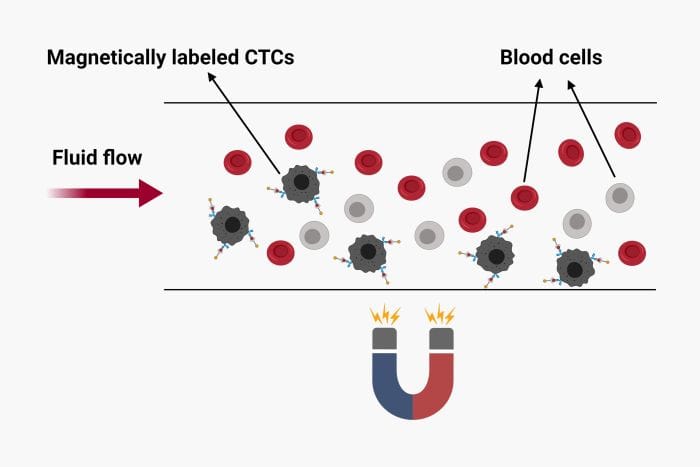
A novel microfluidics approach reported in the Advanced Science journal targets the direct protein analysis of the CTCs to outline the patient’s susceptibility to treatment. The microfluidic chip was used to capture CTCs from whole blood as well as quantifying the secretion level of granulocyte growth stimulating factor (G-CSF).
“In contrast to alternative methods, our platform enables the simultaneous analysis of multiple membrane proteins and secreted factors by immunohistochemistry and bead-based sandwich immunoassays. Since we used magnetic beads that were co-immobilized with the CTCs in miniature chambers, we were able to measure secreted molecules with unprecedented sensitivities.”, the authors explained.

Reproduced under Creative Commons License
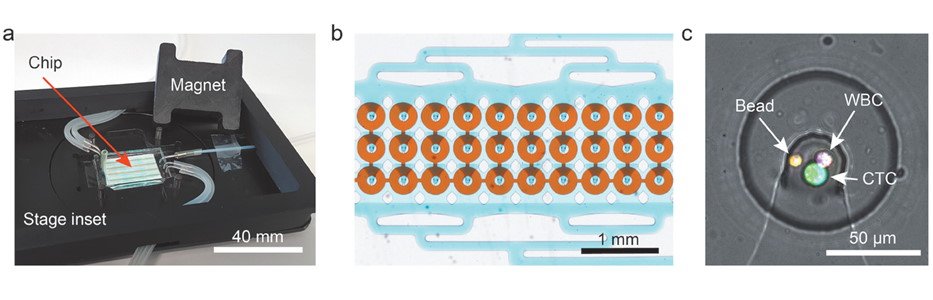
The proposed microfluidic device for CTC isolation and protein quantification was made of PDMS and included two layers separated by a thin PDMS membrane. The top layer consisted of fluidic microchannels and 1152 traps to capture the CTCs while the bottom layer served as a valve to hold the captured cells. The sample was first introduced to the microfluidic device and was optimized for capture efficiency which resulted in up to 95% isolation efficiency. Next, the cells were incubated with magnetic beads functionalized with antibodies against human G-CSF. The valve was then used to ensure the proximity of the magnetic beads to the cells during the incubation. The incubation was followed by on-chip immunostaining using an antibody cocktail. The microchip was then fluorescently imaged to quantify the secretion level of G-CSF as well as other membrane proteins such as HER-2, CD45, and EpCAM.
The microfluidic chip that was proposed for isolation of CTCs and quantifying the protein secretion level was tested with five different cancer cell types and could process the whole blood in 5-6 hours with no requirement for pretreatment. The authors envision a reduction in processing time by parallelization and automation of the steps. The microfluidic device is believed to give a better understanding of the metastases process if the method is expanded to cover a wider range of secreted proteins.
Read the original research article: Quantification of Protein Secretion from Circulating Tumor Cells in Microfluidic Chambers

Pouriya Bayat
Pouriya is a microfluidic production engineer at uFluidix. He received his B.Sc. and M.A.Sc. both in Mechanical Engineering from Isfahan University of Technology and York University, respectively. During his master's studies, he had the chance to learn the foundations of microfluidic technology at ACUTE Lab where he focused on designing microfluidic platforms for cell washing and isolation. Upon graduation, he joined uFluidix to even further enjoy designing, manufacturing, and experimenting with microfluidic chips. In his free time, you might find him reading a psychology/philosophy/fantasy book while refilling his coffee every half an hour. Is there a must-read book in your mind, do not hesitate to hit him up with your to-read list.
Microfluidic approaches allow gentle isolation of live cells and thus enable many downstream analyses that rely on captured live CTCs.
Learn more about the techniques used in microfluidics for isolating circulating tumor cells (CTCs) as potential determinants of cancer prognosis.
Personalized cancer therapy is a strategy to predict which patients are more likely to respond to specific cancer therapies. Learn how microfluidics can help.