Microfluidic techniques for isolation, detection, and analysis of exosomes
What are exosomes?
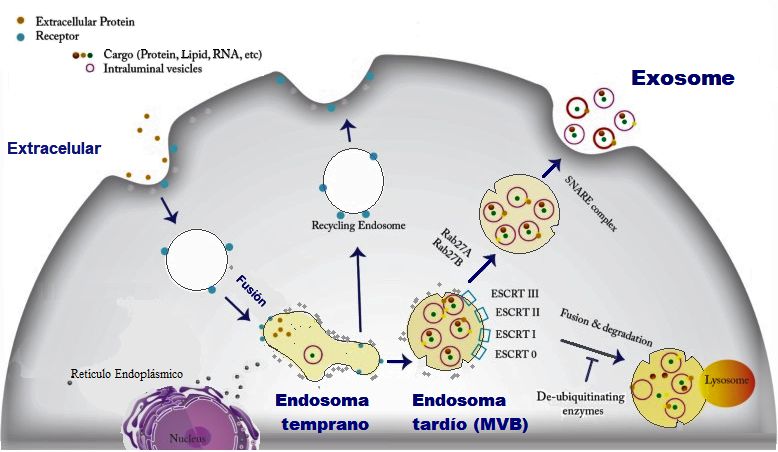 Extracellular vesicles are the cargos that the cells secret to their surroundings and can include apoptotic bodies, microvesicles, and exosomes. These vesicles are different in their origin, size, molecular pathways, and their cargo.
Extracellular vesicles are the cargos that the cells secret to their surroundings and can include apoptotic bodies, microvesicles, and exosomes. These vesicles are different in their origin, size, molecular pathways, and their cargo.
Among different types of extracellular vesicles, exosomes have gained much more attention. These vesicles are different than the cell’s waste and can be loaded with a wide range of materials such as lipids, messenger RNAs, micro RNAs, double-stranded DNA, lipids, and proteins. Exosomes are normally around 30-150 nm in size.
What makes exosomes important?
Exosomes are perceived as a means of communication and molecular transport between cells. This gives exosomes far more significance than what they were thought of in the past and makes them an ideal target for both therapeutic and diagnostic applications. For example, exosomes secreted from stem cells can be a potent source for healing and repairing damaged tissues. On the diagnostic side, it is thought that the exosomes can carry biomarkers from cancerous and can be potentially used as a reliable material for tumor liquid biopsy.
What is challenging in working with exosomes?

Exosome samples – IBM research
Despite the promises of exosomes for disease treatment as well as serving as diagnostic markers, they have not yet found their place in the market. This can be related to the challenges of exosome-related research. The small size of the exosomes (~30-150 nm) and the limited concentration in the bodily fluids impose major challenges for separating them from other analytes. Also, the lack of specific biomarkers and cumbersome characterization in conventional settings make exosomal research less efficient.
Conventionally, exosomes are isolated by density, size, or function using the following methods:
– Ultracentrifugation
– Ultrafiltration
– Density gradient
– Immunoaffinity
– Size exclusion chromatography
– Precipitation
These methods, however, are not able to process large volumes and lack precision. Also, multiple centrifugation steps can damage the exosomes. Microfluidics has gained attention due to an ever-increasing need for efficient and easy-to-use methods for studying exosomes.
How can microfluidic technology be used for exosome research?
Microfluidic chips are made of microchannels and microchambers that are excellent for manipulating micron and submicron particles and small sample volumes. Many microfluidic device designs have been used so far for studying exosomes which have shown high levels of purity and sensitivity as well as low costs and ease of operation compared to conventional methods. Microfluidic chips for exosome studies can be classified into the following applications:
– Isolation
– Detection
– Analysis
Microfluidic isolation of exosomes
As the field of exosome research grows and its application in therapeutics as well diagnostics become more evident, the need for more advanced and easy to use methods for isolation of exosomes also increases. Over the past decade, microfluidics has shown great promises in developing chips to efficiently and affordably isolate exosomes.
Microfluidics can benefit from the same approaches for cell sorting to tailor them for isolating exosomes. Some of these approaches include:
– Immunoaffinity based capturing
– Microfluidic filtration and trapping
– Acoustofluidic isolation
– Deterministic lateral displacement (DLD)
– Inertial microfluidics
More details about these methods can be found under microfluidic cell sorting.
Microfluidic detection of exosomes
Microfeatures in Microfluidics devices can be fabricated so small that along with the possibility of controlling the fluid flow in micro-scale makes it possible to detect exosomes on-chip. For example, a detection microchamber could be integrated with an isolation microdevice to detect the isolated exosomes. Some of the methods that have been embedded on microfluidic chips for detection of exosomes are listed here:
– Fluorescence detection
– Colorimetric detection
– Miniaturized nuclear magnetic resonance
– Magnetic nanoparticle immunolabeling
– Electrical detection

Reproduced under Creative Commons Attribution 4.0 – Ramshani et al., 2019.
Microfluidic analysis of exosomes
Microfluidic chips are modular meaning that they are capable of being integrated to perform a complex and multi-step task. This integration potential is beneficial in increasing throughput and reducing cross-contamination. The chips can be connected to each other via tubings to decrease the off-chip sample preparation as much as possible which in turn reduces the cross-contamination and omits the need for highly skilled personnel. Microfluidic technology can be combined with a wide variety of methods for the on-chip analysis of exosomes. This is of the utmost importance when these chips are designs for point of care applications or clinical settings. Some of the approaches that have been used for exosome analysis using microfluidic chips can be seen in the following. More details about the microfluidic analysis of exosomes can be found in the articles mentioned in the further reading section.
– Overall exosome level
– Detection of disease-specific subpopulations of exosome
– Quantification of intravesicular proteins
– Exosomal mRNA analysis
Further Reading
- Technical challenges of working with extracellular vesicles
- Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy
- Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine
- Microfluidic on-demand engineering of exosomes towards cancer immunotherapy
- Microfluidic engineering of exosomes editing cellular messages for precision therapeutics


