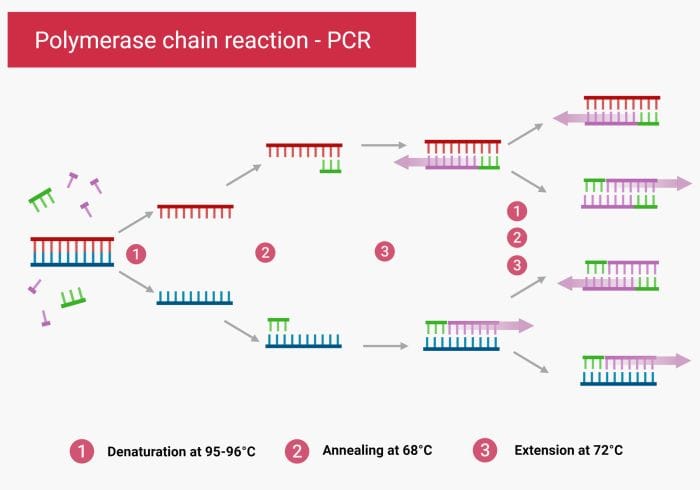
Microfluidic methods for nucleic acid amplification
Lab on a chips are handheld microfluidic devices that are destined to integrate everyday lab routines. They can be compartmentalized to perform these tasks in microchambers connected with microchannels manufactured using Microfluidics fabrication processes. For example, a lab on a chip device for a molecular biology application might have three sections. One section for extracting target analytes from a raw sample connected a sample preparation unit which capable of performing enzymatic reactions or amplification. These can be followed by a third part as a readout system that allows the prepared sample to be analyzed, for instance, an electrophoresis module.
A crucial task for sample preparation is nucleic acid amplification that is needed in many molecular biology experiments. Since its emergence, various nucleic acid amplification techniques have been developed. In general, they are classified as isothermal and non-isothermal methods. As the name implies, non-isothermal approaches require temperature changes during the amplification process while isothermal techniques do not. Microfluidic technology is adjustable to both these methods to allow rapid amplification. Microfluidics, compared to conventional methods, carries several advantages such as affordability and modularity. Also, the chips are capable of operating at the point of care while common machines such as PCR are not. Besides, these microfluidics chips need lesser reagents and operate faster. The risk of contamination while handling the samples is lower with microfluidics chips.
Why using a microfluidic device for nucleic acid amplification?
Nucleic acid amplification is a strong tool for diagnostics. Nucleic acid amplification tests allow very small amounts of a target sequence to be identified by amplifying the signal. They can provide quantitative data that can be used for the diagnosis of pathogenic and infectious diseases. There are hundreds, if not thousands, of methods, protocols, and devices such as thermocyclers for amplifying nucleic acids. So why should one want to take a microfluidic approach for nucleic acid amplification?
Nucleic acid amplification can be a crucial element for many point-of-care assays where a drop of blood or other bodily fluids can be used to identify a target pathogen. Point-of-care devices are aimed at delivering affordable healthcare products and services to the patients at the time and place of need. Point-of-care diagnosis can benefit from nucleic acid amplification tests. Commercially available nucleic acid amplification devices like PCR machines are not suitable for point-of-care applications. Oftentimes, they are bulky and expensive. Also, they require a trained operator for sample preparation and data analysis. These are not ideal for point-of-care applications where cost and easy operation are of high importance. Microfluidics, however, can overcome these barriers.
Microfluidics chips are easily mass-producible at an affordable cost. They can be automated omit the need for trained operators. Moreover, microfluidic chips can be integrated with a wide variety of sample preparation, sensing, analysis, and communication modules.
Polymerase Chain Reaction (PCR) on chip
Conventional Polymerase Chain Reaction (PCR) is a non-isothermal approach. It requires changing the temperature in each cycle to trigger denaturing, annealing, and extension. Therefore, the reagents in the microfluidics chip need to undergo several rounds of thermocycling. In general, there are two methods for performing a PCR reaction on a chip. The reagents are either stationary or flowing during the process (Transient PCR or time-domain PCR and flow-through PCR or space domain PCR).
Flow-through or space-domain PCR-on-chip
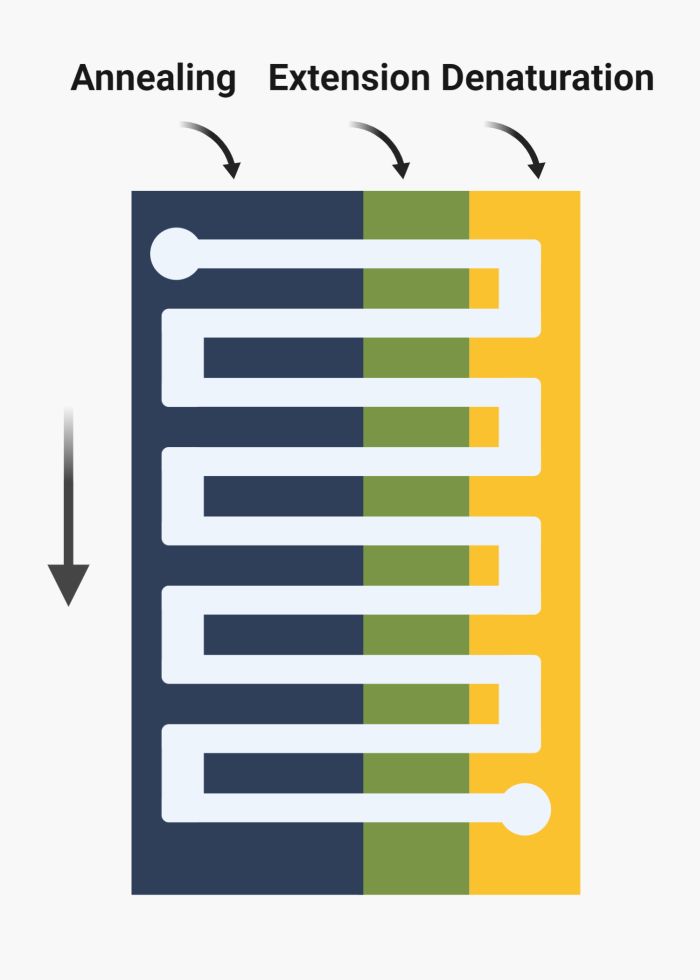 In the flow-through or space domain method, the reagents undergo periodic heating and cooling by cycling between different temperature zones. Here, there is no need for a temperature control unit; two chambers with different temperatures suffice. Two common methods in flow-through nucleic acid amplification are oscillating-flow and serpentine channels. In oscillating-flow, the microfluidics channel is a simple straight channel placed on various temperature zones. The chemicals cycle back and forth between the temperature zones using a pump. Parallel channels can be used to increase throughput. The oscillatory motion can simply be controlled to allow longer residence in any of the temperature zones. In serpentine-channel methods, instead of oscillating, the chemicals flow through a meandering channel. The microfluidics channel passes through zones of high and low temperatures to mimic a PCR cycle. Here, the flow-rate determines the residence time in temperature zones. The length of the channel can change to increase/decrease the number of cycles. For example, 30 turns for 30 PCR cycles.
In the flow-through or space domain method, the reagents undergo periodic heating and cooling by cycling between different temperature zones. Here, there is no need for a temperature control unit; two chambers with different temperatures suffice. Two common methods in flow-through nucleic acid amplification are oscillating-flow and serpentine channels. In oscillating-flow, the microfluidics channel is a simple straight channel placed on various temperature zones. The chemicals cycle back and forth between the temperature zones using a pump. Parallel channels can be used to increase throughput. The oscillatory motion can simply be controlled to allow longer residence in any of the temperature zones. In serpentine-channel methods, instead of oscillating, the chemicals flow through a meandering channel. The microfluidics channel passes through zones of high and low temperatures to mimic a PCR cycle. Here, the flow-rate determines the residence time in temperature zones. The length of the channel can change to increase/decrease the number of cycles. For example, 30 turns for 30 PCR cycles.
Transient or time-domain PCR-on-chip
In the stationary mode, the reagents remain stagnant in a microchamber throughout the process. A temperature control unit handles heating up and cooling down the reagents. After the cycles are finished, the PCR products are analyzed in the downstream of the microfluidic channel or flushed out for off-chip analysis. In transient methods, the reagents reside inside microchambers. There are various ways of heating and cooling the chips. The heaters can be placed around the chambers to heat up and cool down the reagents. Transferring the heat from an external heater to the chambers can be time-consuming and inefficient. Heating up and cooling down the chamber could be time-consuming with an external heater. This can be overcome by using internal electrode heaters. The electrodes are placed inside the chambers and connect to a power supply to heat up the chambers. A common method is printing electrodes on the glass slides and bonding them to the chips. The electrodes are normally made of Platinum (Pt) covered by a layer of gold (Au). The glass is first coated with a photoresist and patterned using a photomask. Then an adhesive layer of Titanium lies on the patterned photoresist followed by deposition of a Pt and Au layer. The photoresist then should be developed in the developer solution to lift the Pt and Au layers off (in non-electrode areas). This glass bonds to the microchannel to form the on-chip heating module. The electrodes connect to a power supply. The temperature changes by altering the current and voltage.
Isothermal microfluidic approaches for nucleic acid amplification
Although the most popular amplification method is thermocyling PCR, the necessity of using a thermocycler motivates many researchers to use isothermal techniques. As the name suggests, isothermal methods operate at a constant temperature. The isothermal approaches integrated with microfluidics include but are not limited to:
– Loop-mediated isothermal amplification (LAMP)
– Nucleic-acid sequence-based amplification (NASBA)
– Strand displacement amplification (SDA)
– Rolling circle amplification (RCA)
Further Reading
- Isothermal nucleic acid amplification technologies for point-of-care diagnostics
- Polymerase chain reaction in microfluidic devices
- Integrated microfluidic systems with sample preparation and nucleic acid amplificationMicrofluidic Assembly of pDNA Cationic Liposome Lipoplexes with High pDNA Loading for Gene Delivery
- Microfluidic-based nucleic acid amplification systems in micorbiology
- Miniaturized isothermal nucleic acid amplification, a review
- Nucleic acid amplification using microfluidic systems
uFluidix can help!